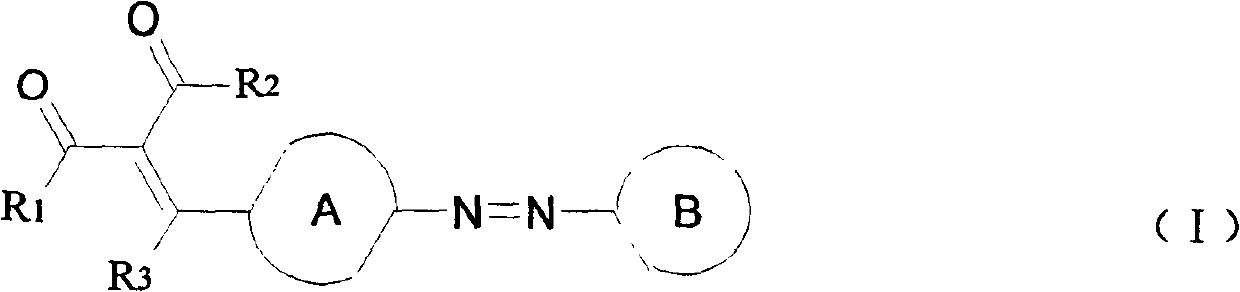
Azo-dye as light storage medium and its synthesis
An optical storage medium, azo dye technology, applied in the direction of azo dyes, monoazo dyes, recording/reproducing by optical methods, etc. The effect of good efficiency, easy control of reaction conditions and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
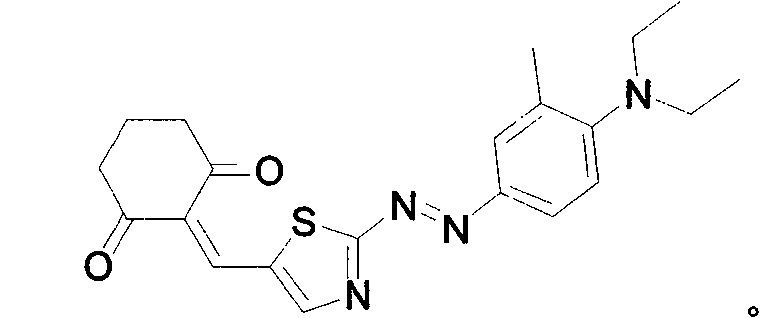
[0016] Embodiment 1, target compound:
[0017]
[0018] resolve resolution:
[0019] 1.28 g of 2-amino-5-formylthiazole was dissolved in 20 ml of 85% concentrated phosphoric acid, cooled to 0-5°C in an ice bath, and then 0.76 g of solid sodium nitrite was added thereto to obtain a diazotization solution.
[0020] Dissolve 1.80g of N,N-diethyl-o-toluidine, 3g of sodium acetate, and 0.3g of urea in 30ml of methanol, cool down in an ice bath to 0-5°C, add the above diazotization solution dropwise, and keep the temperature for 3h. Filter and dry:
[0021]
[0022] Dissolve 1.61g of the above-mentioned product and 0.62g of 1,3-cyclohexanedione in 60ml of absolute ethanol, add a catalyst, heat to reflux, and detect that the end point of the reaction is reached, then cool, and solids are precipitated, filtered and washed three times with ice ethanol, Recrystallize from absolute ethanol, recrystallize again after column chromatography, and obtain the pure dye that conforms to ...
Embodiment 2
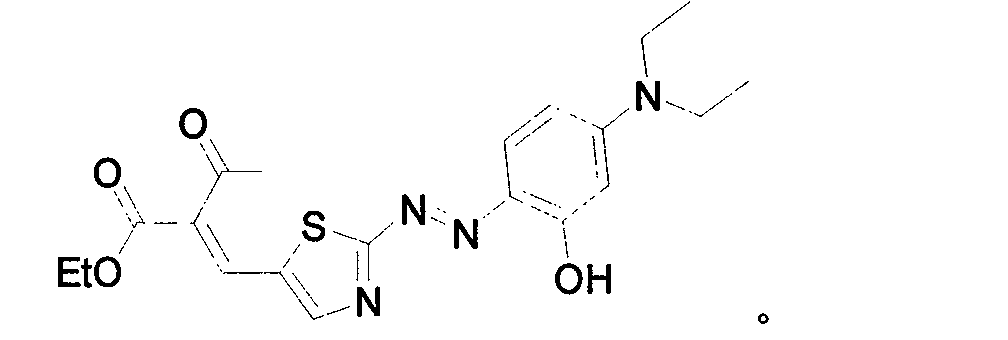
[0027] Embodiment 2: target compound:
[0028]
[0029] resolve resolution:
[0030] 1.28 g of 2-amino-5-formyl thiazole was dissolved in 20 mL of 85% concentrated phosphoric acid, cooled to 0-5°C in an ice bath, and then 0.76 g of solid sodium nitrite was added thereto to obtain a diazotization solution.
[0031] Dissolve 1.80g of N,N-diethyl-m-hydroxyaniline, 3g of sodium acetate, and 0.3g of urea in 30ml of methanol, cool down in an ice bath to 0-5°C, add the above diazotization solution dropwise, and keep the temperature for 3h. Filter and dry to get:
[0032]
[0033] Dissolve 1.61g of the above-mentioned product and 0.62g of ethyl 2-ketobutyrate in 60ml of absolute ethanol, add a catalyst, heat to reflux, and detect that the end point of the reaction is reached, then cool, and a solid precipitates out. After filtering, wash with ice ethanol three times. Recrystallize from absolute ethanol, recrystallize again after column chromatography, and obtain the pure dye t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com