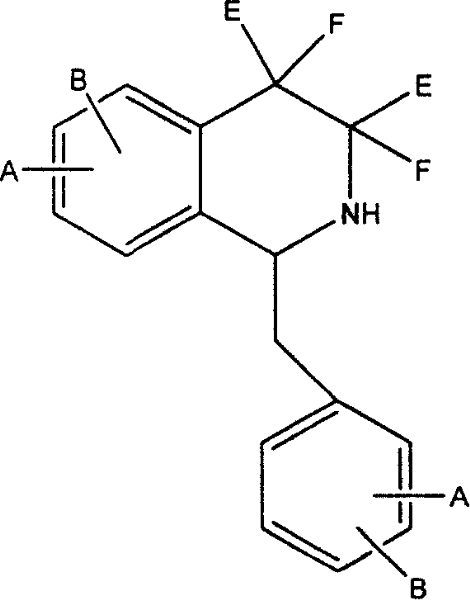
Splitting method of tetrahydroisoquinoline racemes
A technology of racemate and racemate, which is applied in the direction of organic racemization, organic chemical methods, separation of optically active compounds, etc., can solve the problems of limited industrialization, difficulty in obtaining, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Dissolve 38g of tetrahydropapaverine hydrochloride in 500mL of water, add ammonia water to neutralize until neutral, extract with 200ml of dichloromethane×3, combine the organic phases, wash with salt water, dry, filter, concentrate the organic phase to dryness, and wash with isopropanol 500ml was dissolved, and 19g of N-acetyl-L-leucine was added, refluxed, cooled, and crystallized at room temperature overnight. Filtrate, concentrate the filtrate to dryness, add ammonia water to neutralize to neutrality, dichloromethane 150ml×3 extract, combine the organic phases, wash with salt water, dry, filter and concentrate the organic phases to dryness, dissolve with 300ml isopropanol, add N- Acetyl-D-leucine 12g was refluxed, cooled, and crystallized at room temperature overnight. After filtration, the filter cake was recrystallized with acetone to obtain 22g. Neutralize with ammonia water, extract with 300mL of toluene, add ethanol hydrochloride to the toluene layer to pH 1-2...
Embodiment 2
[0042] Dissolve 76g of tetrahydropapaverine hydrochloride in 800mL of water, add 1N sodium hydroxide to neutralize to neutral, extract with 300ml of toluene×3, combine the organic phases, wash with salt water, dry, filter, concentrate the organic phase to dryness, and wash with 500ml of methanol Dissolve, add 38g of N-acetyl-L-leucine, 300ml of ether, freeze and crystallize overnight in the refrigerator. Filtrate, concentrate the filtrate to dryness, add ammonia water to neutralize to neutral, extract with 250ml×3 toluene, combine the organic phases, wash with salt water, dry, filter and concentrate the organic phase to dryness, dissolve with 300ml methanol, add N-acetyl-D- Leucine 26g, ether 100ml, crystallized overnight. After filtration, the filter cake was recrystallized with acetone to obtain 60g. Neutralize with ammonia water, extract with 300mL of toluene, add ethanol hydrochloride to the toluene layer to pH 1-2, and crystallize in the refrigerator overnight to obtain ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com