Method for preparing valienamine
A technology of Jinggangmemamine and metal, applied in the field of preparation of Jinggangmemamine, can solve problems such as being too complicated and unable to be used for mass production and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
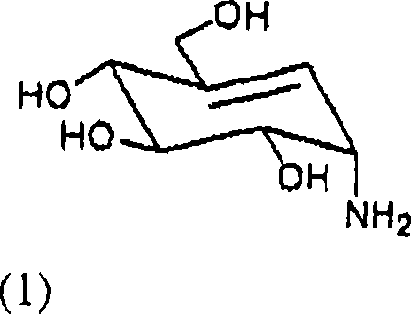
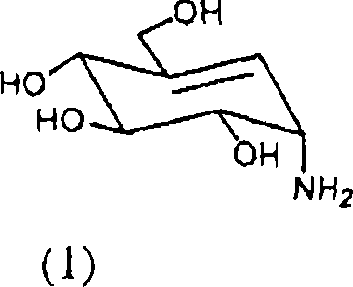
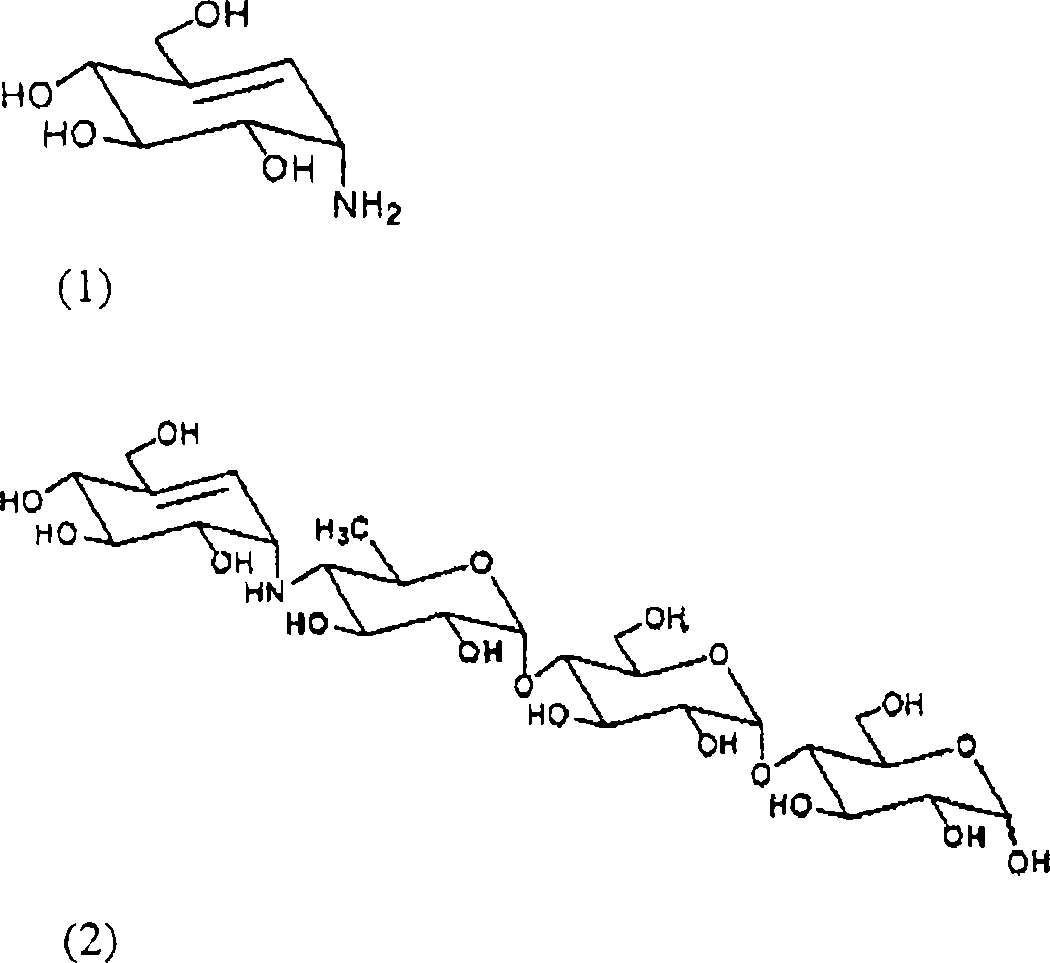
[0035] Embodiment 1: Jinggang mycylamine is prepared by acarbose (1)
[0036] Acarbose (10 g) and sodium hydroxide (9.3 g) were added to water (200 mL), followed by reflux for 48 hours. The reaction mixture was cooled to room temperature, neutralized with 1N hydrochloric acid solution, and then concentrated. The concentrated reaction mixture was purified with cation exchange resin (Amberlite IR-120H) and weak acid cation exchange resin (Amberlite CG-50). As a result, pure mycoenamine (1.1 g) was obtained.
[0037] The hydrogen NMR spectrum of the product (Jinggang mycylamine) formed is as follows:
[0038] 1 H NMR (D 2 O, 300MHz) 3.35(m, 1H), 3.49(m, 2H), 3.80-3.95(m, 2H), 4.02(d, 1H), 5.61(d, 1H)
Embodiment 2
[0039] Embodiment 2: Preparation of Jinggang mycylamine by acarbose (2)
[0040] Acarbose (10 g) and potassium hydroxide (10.5 g) were added to water (180 mL), followed by reflux for 48 hours. The reaction mixture was cooled to room temperature, neutralized with 1N hydrochloric acid solution, and then concentrated. The concentrated reaction mixture was purified with cation exchange resin (Amberlite IR-120H) and weak acid cation exchange resin (Amberlite CG-50). As a result, pure mycoenamine (1.0 g) was obtained.
Embodiment 3
[0041] Embodiment 3: Jinggang mycylamine is prepared by acarbose (3)
[0042]Acarbose (1.0 g) and calcium hydroxide (1.6 g) were added to water (20 mL), followed by reflux for 60 hours. The reaction mixture was cooled to room temperature, then concentrated. The concentrated reaction mixture was purified with cation exchange resin (Amberlite IR-120H) and weak acid cation exchange resin (Amberlite CG-50). As a result, pure mycoenamine (0.12 g) was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com