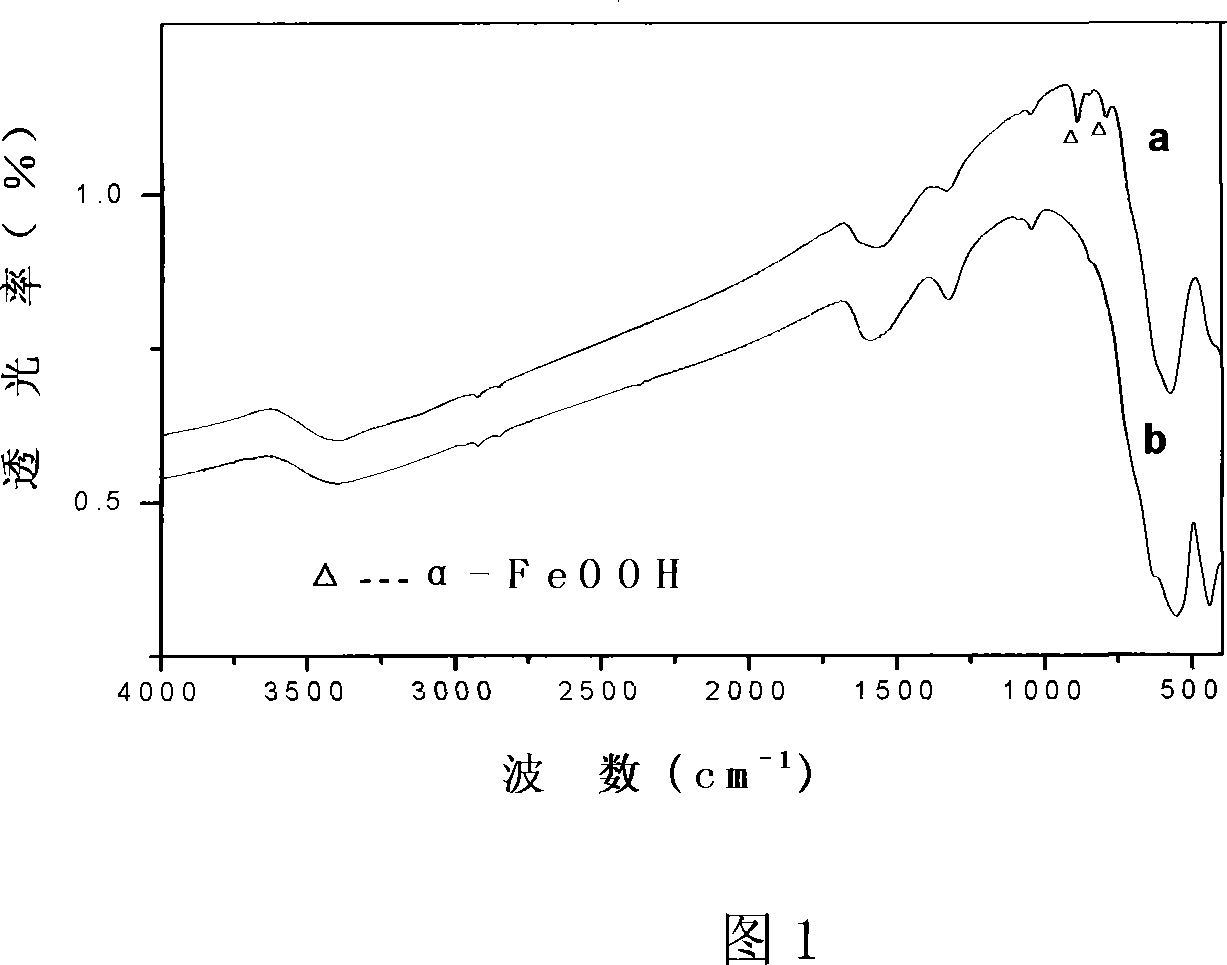
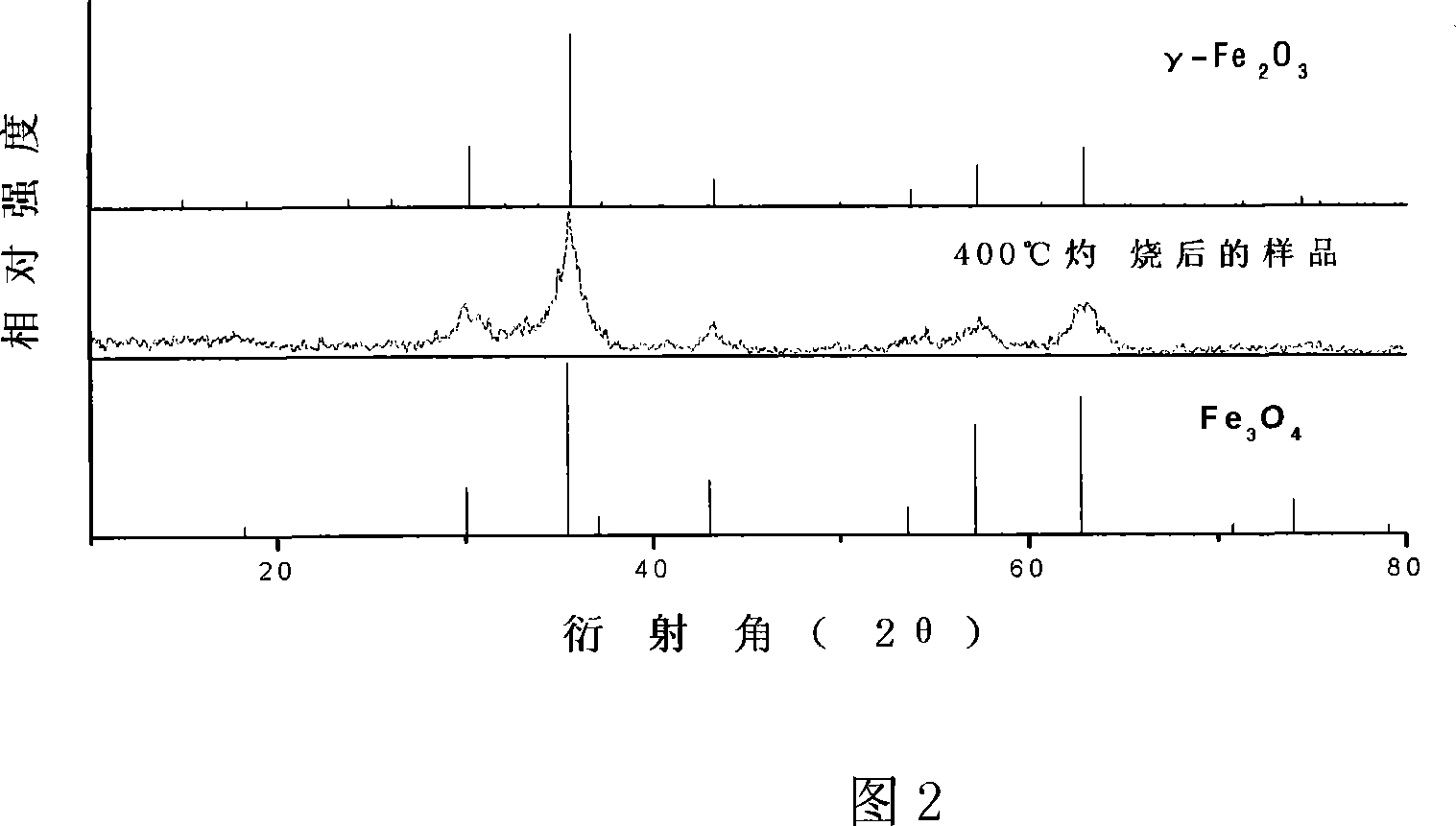
Method for preparing Fe2O3 Nano particles clad by Fe2O3
A nanoparticle, fe2o3 technology, applied in nanotechnology, nanotechnology, nanostructure manufacturing, etc., can solve the problems of complicated coating process and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Preparation of Fe by co-precipitation method 3 o 4 Nanoparticles. Boil in distilled water for 20 minutes, and then pass nitrogen gas for 30 minutes to prepare 25ml of deoxygenated distilled water and 0.85ml of HCl with a concentration of 36% to 38%, mix in a container until the pH of the solution is 1, then add FeCl 3 ·6H 2 O and FeCl 2 4H 2 O makes a solution of iron salts. In the prepared iron salt solution, FeCl 3 ·6H 2 The concentration of O is 1mol / L, FeCl 2 4H 2 The concentration of O is 0.5mol / L. Add 25ml of the iron salt solution dropwise to 250ml of NaOH solution with a concentration of 1.5mol / L under stirring at 15°C. After the addition is completed, continue to react for 30min, and pass nitrogen gas during the whole reaction process. protection, after the reaction is completed, settle under an external magnetic field, remove the supernatant, and then wash the residue three times with deoxygenated distilled water, then wash with ethanol, centrifuge, a...
Embodiment 2
[0021] Boil in distilled water for 20 minutes, and then pass nitrogen gas for 30 minutes to prepare 25ml of deoxygenated distilled water and 0.85ml of HCl with a concentration of 36% to 38%, mix in a container until the pH of the solution is 1, then add FeCl 3 ·6H 2 O and FeCl 2 4H 2 O makes a solution of iron salts. In the prepared iron salt solution, FeCl3 ·6H 2 The concentration of O is 1.28mol / L, FeCl 2 4H 2 The concentration of O is 0.64mol / L. Add 25ml of the iron salt solution dropwise to 250ml of NaOH solution with a concentration of 0.8mol / L under stirring at 30°C, and a black precipitate is formed immediately. After the addition is completed, continue the reaction for 45min. The whole reaction process was protected by nitrogen gas. After the reaction is completed, settle under an external magnetic field, remove the supernatant, and wash the residue three times with deoxygenated distilled water, then wash with ethanol, centrifuge, and vacuum-dry at 50°C to obtain...
Embodiment 3
[0024] Boil in distilled water for 20 minutes, and then pass nitrogen gas for 30 minutes to prepare 25ml of deoxygenated distilled water and 0.85ml of HCl with a concentration of 36% to 38%, mix in a container until the pH of the solution is 1, then add FeCl 3 ·6H 2 O and FeCl 2 4H 2 O makes a solution of iron salts. In the prepared iron salt solution, FeCl 3 ·6H 2 The concentration of O is 1.28mol / L, FeCl 2 4H 2 The concentration of O is 0.64mol / L. Add 25ml of the iron salt solution dropwise to 250ml of NaOH solution with a concentration of 1.0mol / L under stirring at 50°C. After the addition is completed, continue to react for 90min, and pass nitrogen gas during the whole reaction process. protection, after the reaction is completed, settle under an external magnetic field, remove the supernatant, and then wash the residue three times with deoxygenated distilled water, then wash with ethanol, centrifuge, and dry under vacuum at 50 ° C to finally obtain Fe 3 o 4 Predom...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com