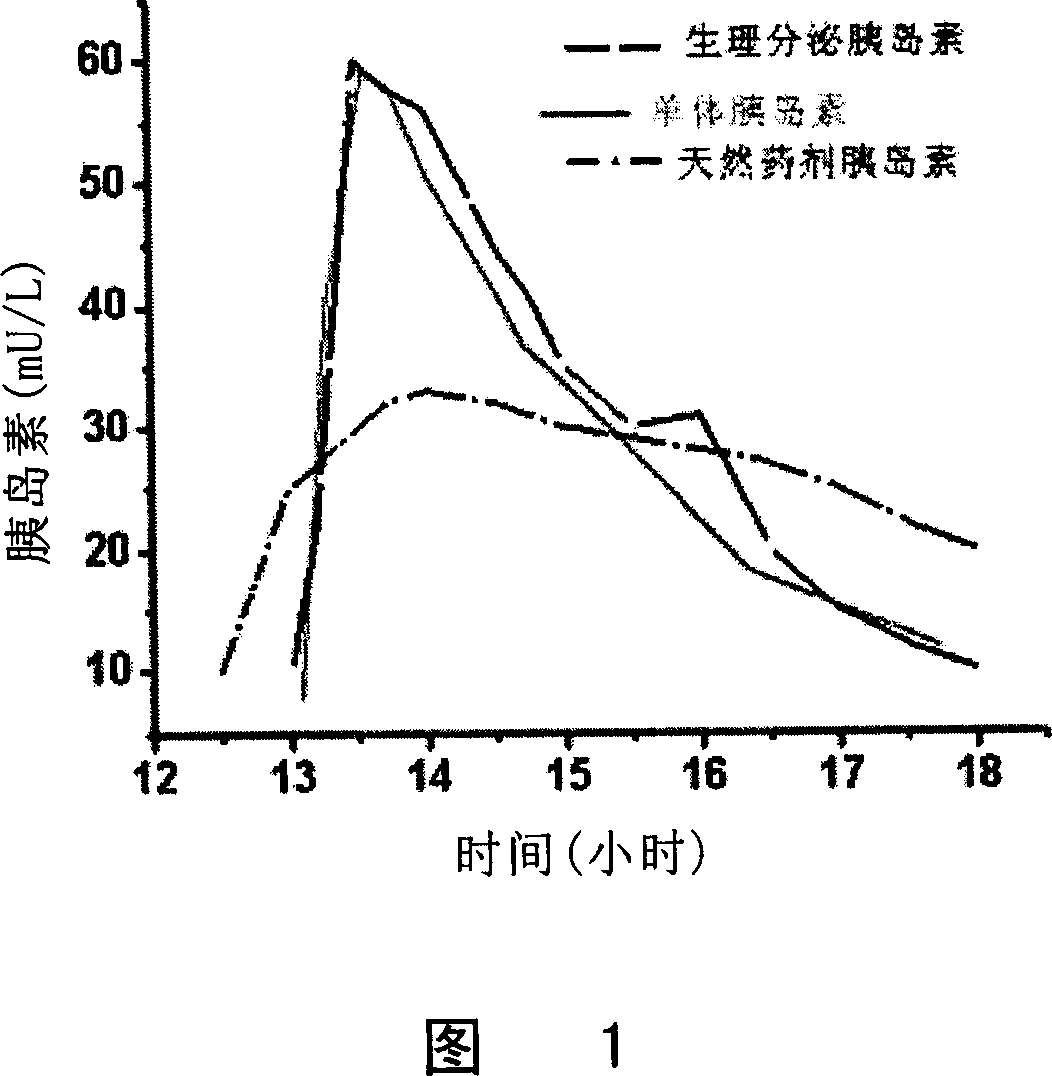
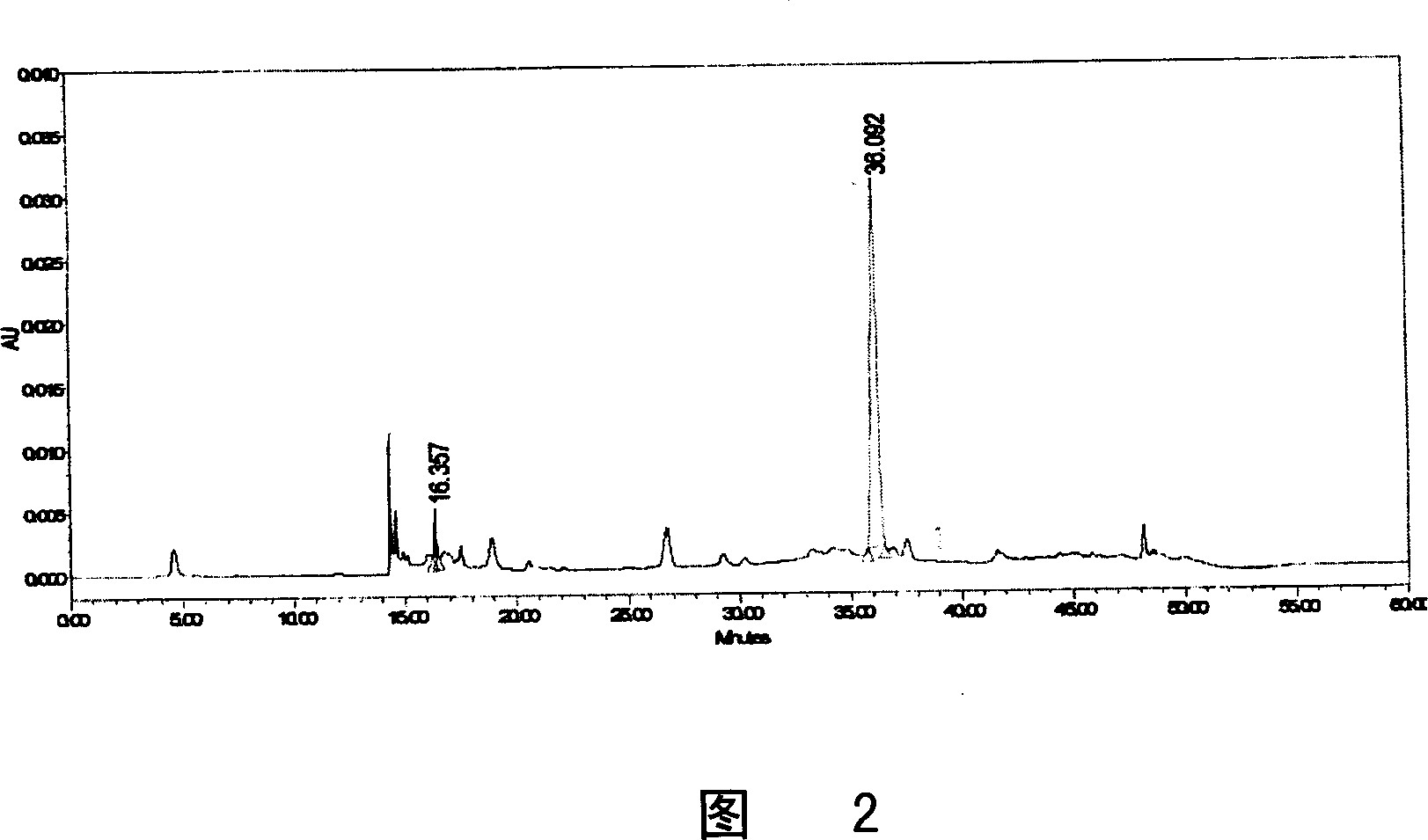
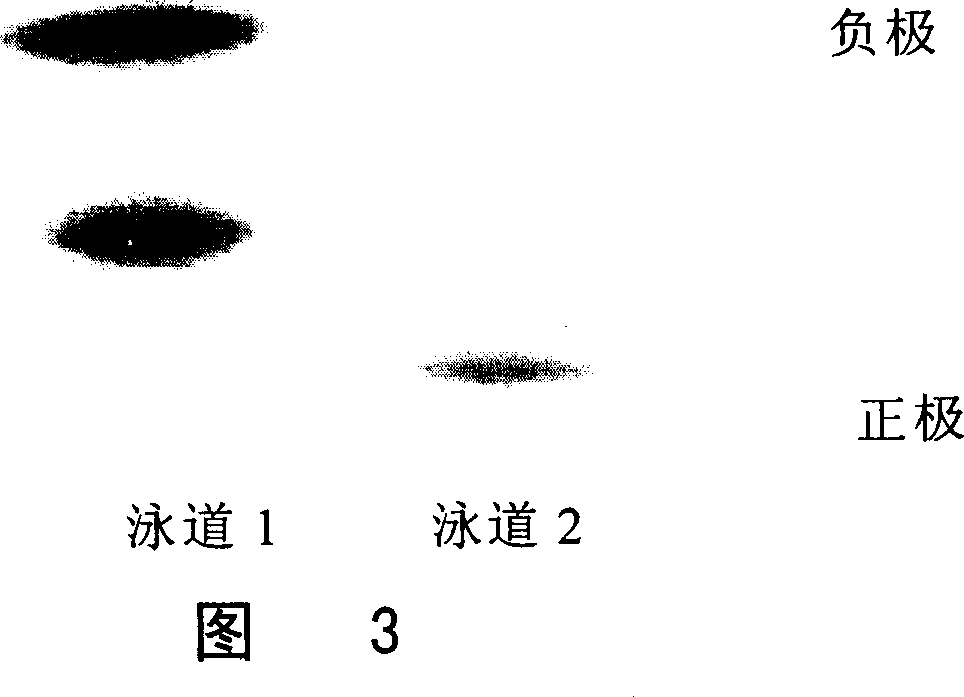
Monomer quick-effective insulin and preparation method and usage thereof
An insulin and monomer technology, applied in the fields of medicine and pharmacy, which can solve the problems of pig insulin shock, excess, and exit from the market.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Genetic engineering to express B22Glu-desB30 insulin
[0108] In this example, the C-terminus of the B chain of B22Glu-desB30 insulin is connected to the N-terminus of the A chain with a linker peptide (such as Ala-Ala-Lys) to form an expression precursor (EPIP), because the structure of the precursor is conducive to the expression of insulin in After biosynthesis, it folds and forms the correct disulfide bonds. Proceed as follows:
[0109] The precursor gene was obtained by DNA synthesis method, and the sequence is as follows;
[0110] ttc gtt aac caa cac ttg tgc ggt tcc cac ttg gtt gag gct ttg tac ttg gtt tgc ggt gaa gaa ggt ttc t tc tac act cct aag gct gct aag ggt att gtcgaa caa tgc tgt acc tcc atc tgc tcc ttg tac caa ttg gaa aac tac tgc aac (SEQ ID NO: 3)
[0111] After adding BamH1 and EcoR1 restriction sites at both ends, the BamH1 and EcoR1 restriction sites were used to clone into pPIC9K (purchased from INVITROGEN) plasmid to obtain pPIC9K / EPIP. pPIC9K / EPIP...
Embodiment 2
[0115] Determination of Self-polymerization Properties of Dezincified Insulin
[0116] In this example, the self-polymerization properties of dezincified insulin were determined by conventional methods.
[0117] Higher concentrations of insulin in neutral solution exist as a mixture of monomers, disomes, and hexasomes. Chromatography with molecular sieves: Superdex 75 (HR 10 / 30) column, mobile phase is phosphate buffer, pH 7.4, flow rate is 0.5ml / min, sample volume is 40 microliters, room temperature, 230nm detection, peptide concentration from Low to high are: 38μM (0.22mg / mL), 75μM (0.43mg / mL), 150μM (0.86mg / mL), 300μM (1.73mg / mL), 600 (3.46mg / mL)μM. The average molecular weight of the mixture is described by the partition coefficient Kd. Kd=(Vr-V 0 ) / (Vc-V 0 ), where Vr is the outflow volume, V 0 is the external water volume, and Vc is the total column bed volume.
[0118] The results show that with the increase of the concentration of dezincified insulin, its retenti...
Embodiment 3
[0121] Determination of Self-polymerization Properties of B22Glu-des-B30 Insulin by Molecular Sieve Column Chromatography
[0122] In this example, the self-polymerization property of the monomeric insulin prepared in Example 1 was measured by conventional methods.
[0123] Except the polypeptide concentration, the implementation conditions are the same as in Example 2. Peptide concentration: 2mg / ml, 5mg / ml, 10mg / ml. As the concentration of the polypeptide increases, the peak time, peak shape and Kd value of B22Glu-desB30 insulin do not change with the concentration of the polypeptide (Figure 5, Table 2 ). This indicated that B22Glu-desB30 insulin exists in the form of monomer at the injection concentration of 500μM (3mg / ml).
[0124] Table 2 B22Glu-desB30 insulin
[0125] Concentration (mg / ml)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com