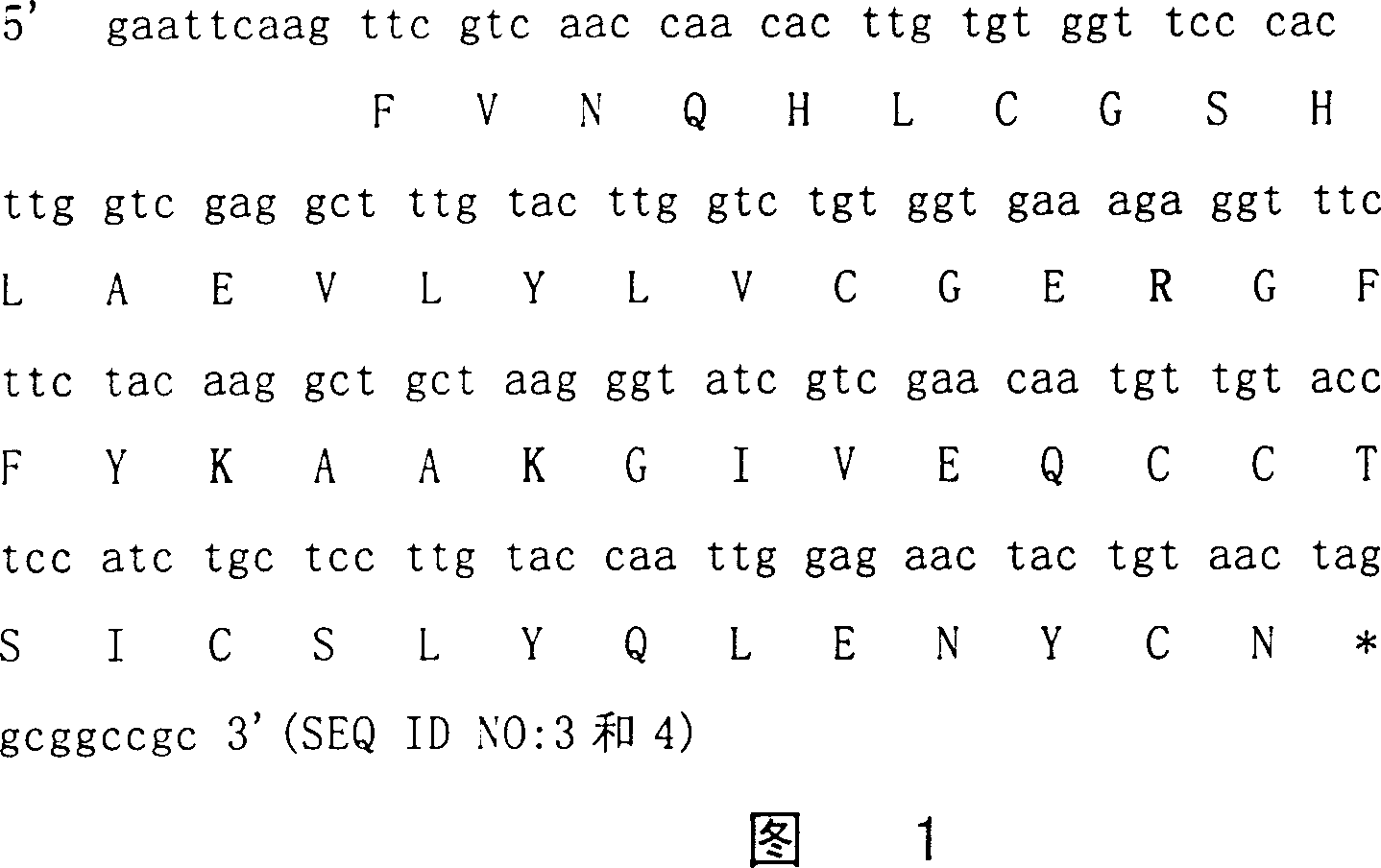B-chain modified monomer quick-acting insulin and method for preparing same
An insulin and monomer technology, applied in the fields of medicine and pharmacy, can solve the problems of difficult large-scale production, complex process flow, reduced immunogenicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Construction of expression vector
[0076] The codon usage of P. pastoris is basically similar to that of S. cerevisiae, but there are some differences in individual amino acids such as Glu [Zhao, X., Huo, K.K. and Li, Y.Y.(2000). Synonymous codon usage in Pichia pastoris Sheng Wu Gong Cheng XueBao16(3):308-11.]. Chemical synthesis of monomeric insulin B following the codon preference of P. pastoris 27 K-DTrI precursor gene fragment (Figure 1).
[0077] Chemically synthesize code B by the same method 1 A B 27 K-DTrI (Figure 2) and (DesB 1 )B 27 DNA of K-DTrI (Fig. 3). Similar to S.cerevisiae, a suitable prepro-leader is helpful for the secretion and expression of insulin precursors in P.pastoris, and the chemically synthesized DNA fragments are cloned into the EcoRI and NotI sites of the pPIC9K plasmid (Invitrogen Company) to form α- Mating factor leader-EAEAYVEFK-MIP expression framework. Among them, the EA-rich spacer peptide (such as EAEAYVEFK, SEQ ID NO: 9) ...
Embodiment 2
[0079] Plasmid transformation and screening of MIP expression strains
[0080] 3ug of pPIC9K / MIP plasmid linearized by Bgl II was electrotransformed into Pichia pastoris GS115(his4) (purchased from NRRL, deposit number NRRL Y-15851, US6,730,499), 0.4cm electric shock cup, 2.4KV, 5.3ms, continuous electric shock Twice, MD plates were coated with transformed yeast cells. After the plasmid pPIC9K / MIP is linearized by Bgl II, after entering the yeast cells, part of it will be re-circularized and integrated into the chromosome through single-point insertion to obtain Mut+ phenotype transformants, and the other part of the linear plasmid will be integrated into the chromosome by substitution to obtain Muts Phenotypic transformants, during which multicopy insertion integrations spontaneously form. Pick 200 single clones from the MD plate and inoculate them on YPD plates containing 0, 0.5, and 4 mg / ml of G418 respectively. The clones that grow colonies on all three plates enter the p...
Embodiment 3
[0082] Shake Flask Fermentation and Purification of Insulin Precursor
[0083] Inoculate high-expressing MIP yeast strains into 100 ml of YPD medium, culture in a 500 ml Erlenmeyer flask at 30°C for 48 hours, let the cells settle after standing, pour off the supernatant, and then add 100 ml of MM medium (containing 1% methanol) , continue culturing at 30°C for 96 hours, add methanol once every 24 hours, and collect the fermentation broth by centrifugation after the fermentation is completed.
[0084] The hydrophobic adsorption column XAD-7 (purchased from Sigma Company) has good adsorption capacity for MIP, and can remove inorganic salts, most polysaccharides, pigments and miscellaneous proteins in the fermentation broth. The XAD-7 column was washed with methanol at 5Vc (that is, 5 times the total column bed volume, the same below), and balanced with 10Vc water, then centrifuged at 10Vc to decellularize the fermentation broth, then washed with 10Vc water, 2Vc 15% ethanol conta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com