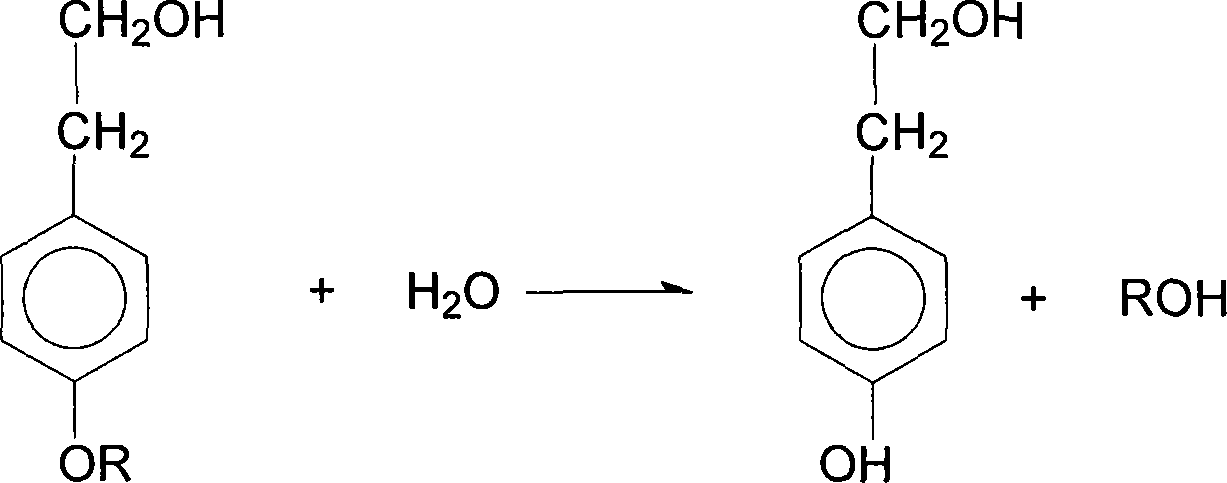
Process of preparing beta-p-hydroxyphenyl ethanol
A technology of p-hydroxyphenylethanol and p-alkoxybenzene, which is applied in the direction of hydrolysis preparation, etc., can solve the problems of complex process, high risk of use, and difficult separation of by-products, and achieve the effect of simple operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Stir the crude p-methoxyphenylethanol to raise the temperature under reduced pressure, rectify under reduced pressure through a rectification column, and collect fractions at 150°C-230°C at a vacuum degree of -0.085MPa. The content of p-methoxyphenethyl alcohol after rectification is more than 98% through gas chromatography analysis, and its single impurity is not higher than 1.0%.
[0024] According to the molar ratio of p-methoxyphenethyl alcohol: hydrochloric acid: water: dichloromethane = 1: 0.30: 1.7: 1.5, the rectified p-methoxyphenethyl alcohol, hydrochloric acid and dichloromethane are mixed, wherein the hydrochloric acid is pre-configured into a 3% aqueous solution, and then add the remainder of water.
[0025] Carry out the hydrolysis reaction at 30°C for 4 hours, and control the vacuum degree at -0.03~-0.05MPa. After the hydrolysis reaction is completed and the vacuum is broken, the temperature is lowered to 0°C, and an appropriate amount of seed crystals are...
Embodiment 2
[0027] Stir the crude p-ethoxyphenethyl alcohol, raise the temperature under reduced pressure, and rectify under reduced pressure through a rectification column, and collect fractions at 150°C-230°C when the vacuum degree is greater than -0.085MPa. The content of p-ethoxyphenethyl alcohol after rectification is more than 98% through gas chromatography analysis, and its single impurity is not higher than 1.0%.
[0028] P-ethoxyphenethyl alcohol: sulfuric acid: water: dichloroethane=1: 0.35: 1.8: 1.8 according to the molar ratio, the rectified p-ethoxyphenethyl alcohol, sulfuric acid and dichloroethane are mixed, wherein the sulfuric acid Pre-configured as a 4% aqueous solution, and then add the balance of water.
[0029] Carry out the hydrolysis reaction at 60°C for 2 hours, and control the vacuum at -0.04~-0.06MPa. After the hydrolysis reaction is completed and the vacuum is broken, the temperature is lowered to 5°C, and an appropriate amount of seed crystals are added to the ...
Embodiment 3
[0031] Stir the crude p-isopropoxyphenylethanol to increase the temperature under reduced pressure, and rectify it through a rectification column under reduced pressure, and collect fractions at 150°C-230°C when the vacuum degree is greater than -0.085MPa. The content of p-isopropoxyphenethyl alcohol after rectification is more than 98% through gas chromatography analysis, and its single impurity is not higher than 1.0%.
[0032] According to the molar ratio of p-isopropoxyphenethyl alcohol: hydrochloric acid: water: chloroform = 1: 0.4: 2.0: 1.7, the rectified p-isopropoxyphenethyl alcohol, hydrochloric acid and chloroform are mixed, wherein the hydrochloric acid is pre-configured to 10 % aqueous solution, and then add the remainder of water.
[0033] Carry out the hydrolysis reaction at 50°C for 5 hours, and control the vacuum degree at -0.04~-0.06MPa. After the hydrolysis reaction is completed and the vacuum is broken, the temperature is lowered to 2°C, and an appropriate a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com