Mixture solution containing conjugate of transferrin and quercetin and use thereof
A technology of transferrin and mixed solution, which is applied in the direction of medical preparations containing active ingredients, medical preparations with non-active ingredients, drug combinations, etc., can solve the problems of cell toxicity, etc. Mild, less toxic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
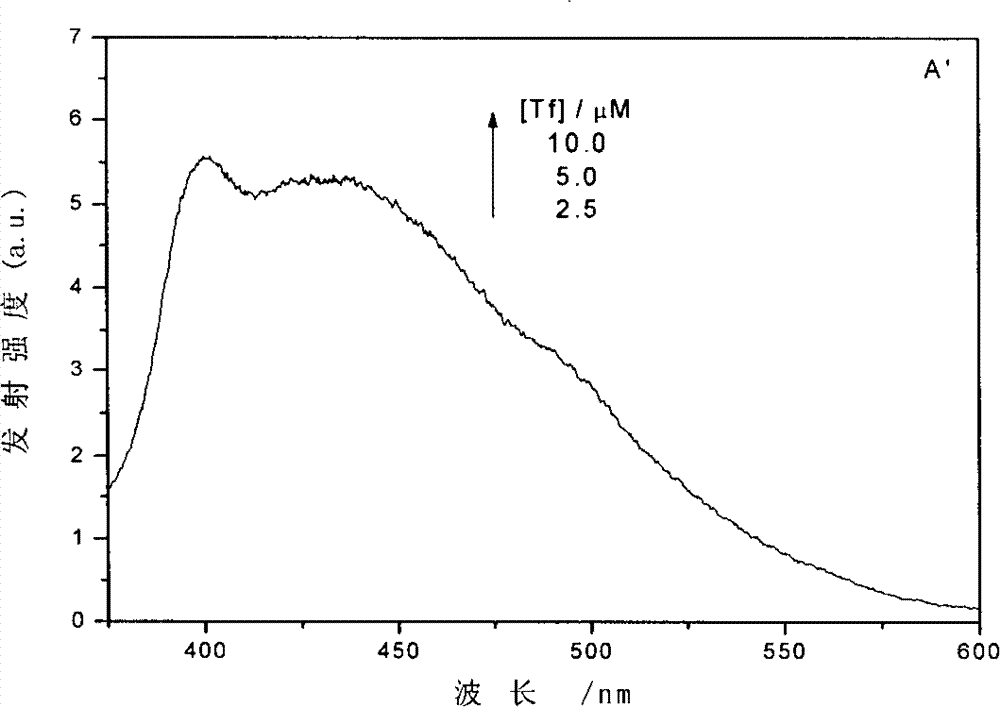
[0025] Add 0.2ml 10×10 to a 5.00ml volumetric flask -4 M quercetin in phosphate buffer solution (pH 7.4), then add 0.4ml 1.25×10 -4 M human transferrin (purchased from Sigma-Aldrich, T3309) in phosphate buffer solution (pH 7.4), the molar concentration ratio of human transferrin and quercetin in the mixed solution is 1:4; The solution was diluted to a quercetin concentration of 40 μM; the mixed solution was shaken evenly and left to stand for 12 hours, and the transferrin reacted with the quercetin to obtain a mixed solution containing a conjugate of the transferrin and quercetin. After UV-Vis absorption spectroscopy analysis, the results are as follows: figure 1 As shown in A, a new absorption peak appeared at 323nm, indicating that quercetin and transferrin interacted to form a conjugate, resulting in characteristic absorption.
[0026] Similarly, after preparing quercetin and human transferrin with pH 4.8 phosphate buffer solution respectively, and then mixing them, the m...
Embodiment 2
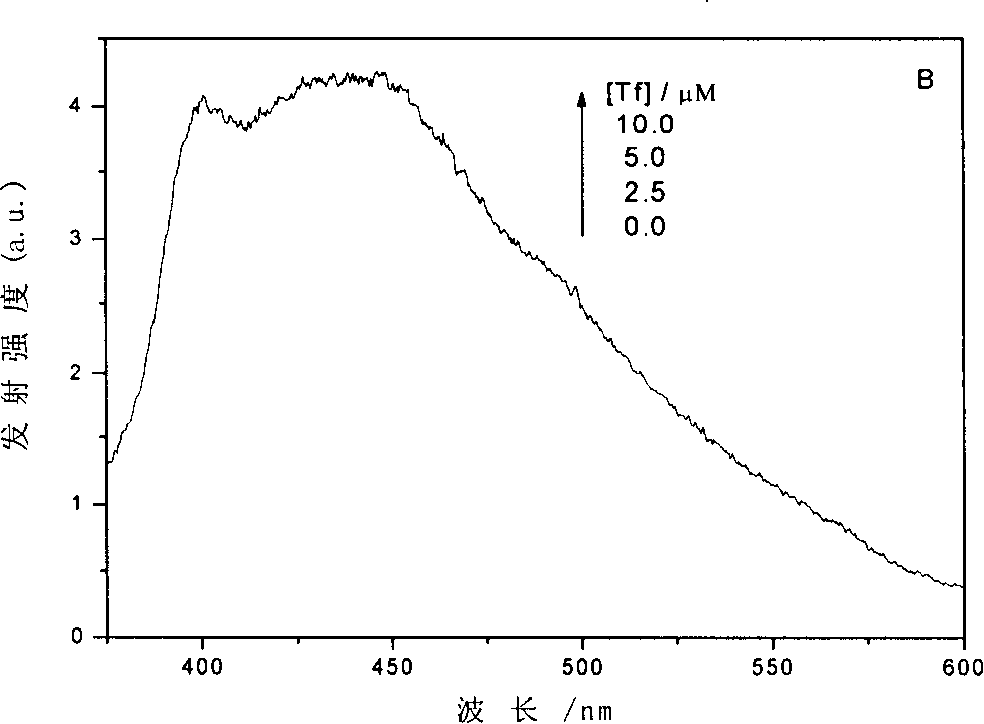
[0030] Add 0.2ml 10×10 to a 5.00ml volumetric flask -4 M quercetin in phosphate buffer solution (pH 6.8), then add 0.4ml 1.25×10 -4Phosphate buffer solution (pH 6.8) of M human transferrin (purchased from Sigma-Aldrich Company, T3309), the molar concentration ratio of human transferrin and quercetin in the mixed solution is 1:4; The solution was diluted to a quercetin concentration of 40 μM; the mixed solution was shaken evenly and left to stand for 12 hours, and the transferrin reacted with the quercetin to obtain a mixed solution containing a conjugate of the transferrin and quercetin. The same results as in Example 1 were obtained by UV-visible absorption spectrum, fluorescence spectrum and circular dichroism analysis, that is, the combination of the two under neutral conditions.
Embodiment 3
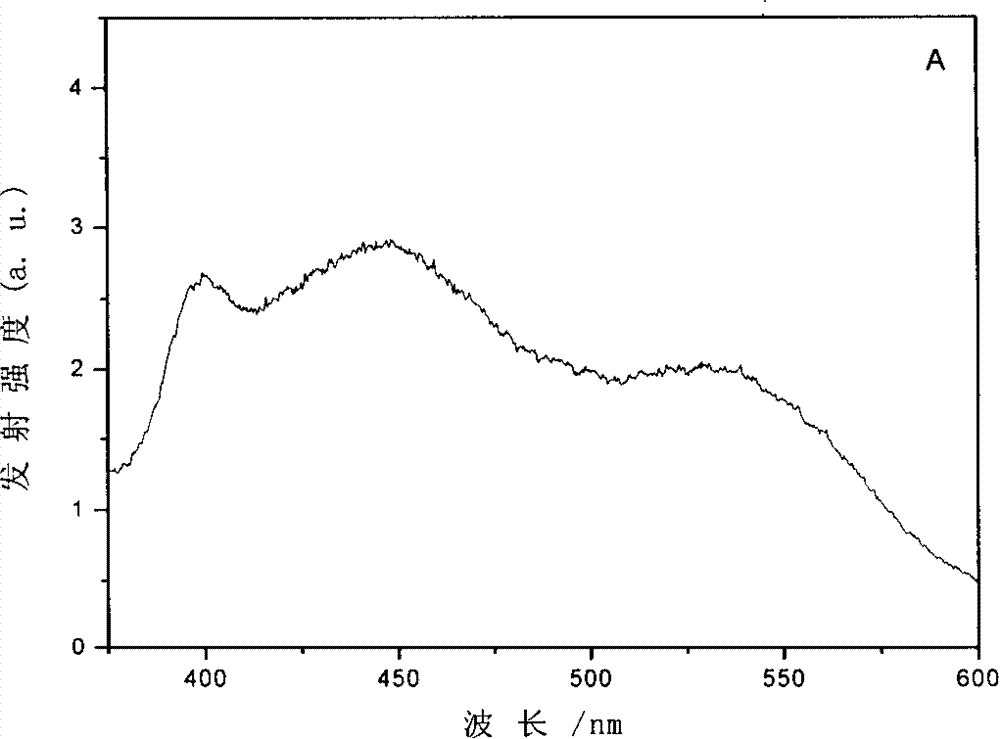
[0032] Add 0.2ml 10×10 to a 5.00ml volumetric flask -4 M quercetin in phosphate buffer solution (pH 7.8), then add 0.4ml 1.25×10 -4 Phosphate buffer solution (pH 7.8) of human transferrin (purchased from Sigma-Aldrich Company, T3309), the molar concentration ratio of human transferrin and quercetin in the mixed solution is 1:4; The solution was diluted to a quercetin concentration of 40 μM; the mixed solution was shaken evenly and left to stand for 12 hours, and the transferrin reacted with the quercetin to obtain a mixed solution containing a conjugate of the transferrin and quercetin. Through the analysis of ultraviolet-visible absorption spectrum, fluorescence spectrum and circular dichroism spectrum, the same result as in Example 1 was obtained, that is, the combination of the two under neutral conditions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com