Clarification method and apparatus for material contaminated with heavy metals
A technology for pollutants and heavy metals, which is applied in the restoration of polluted soil and the removal of solid wastes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0203] [Example 1] Electrolytic extraction and removal test of lead-contaminated soil
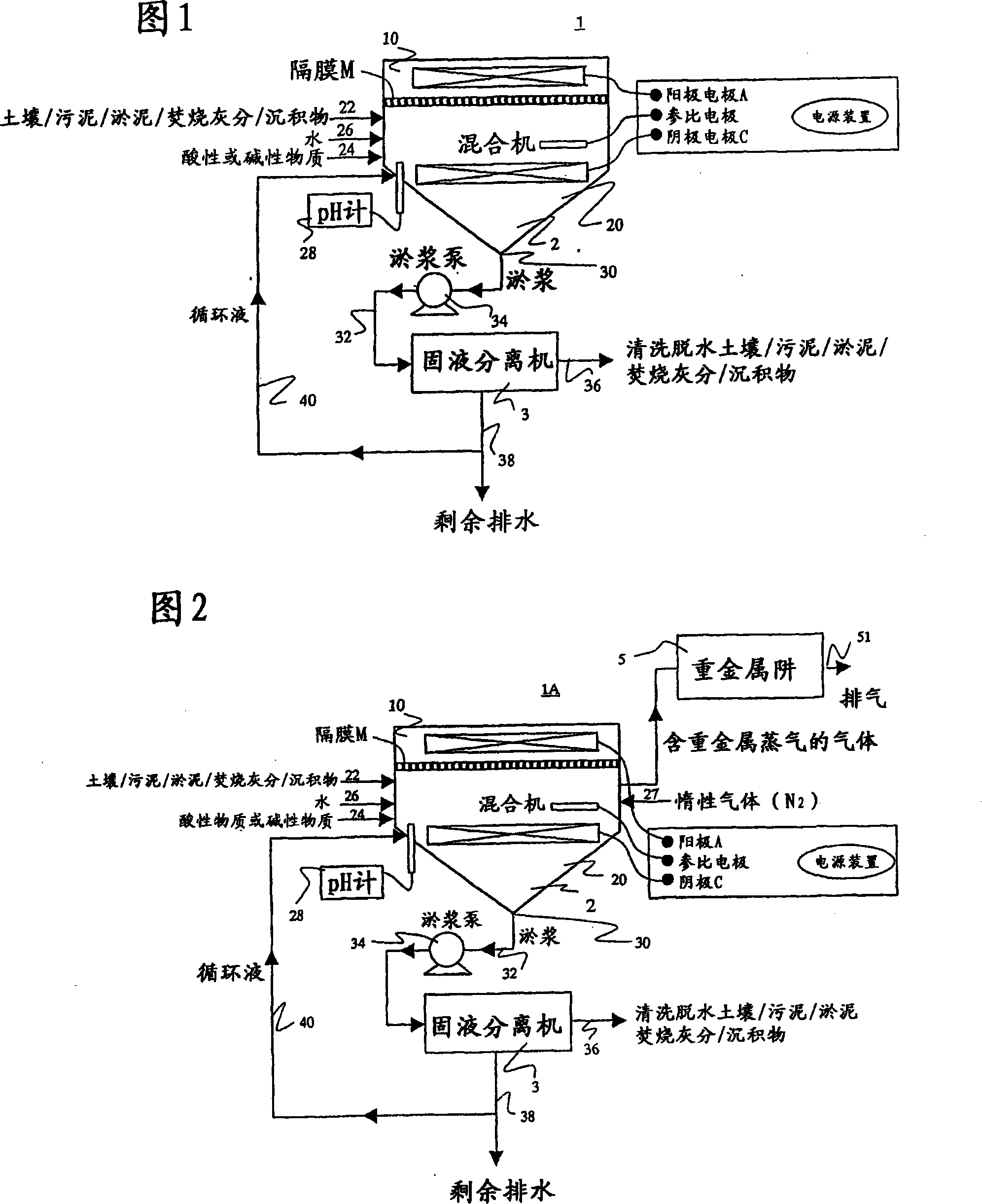
[0204] As shown in Figure 1, a cation exchange membrane (NEOSEPTA CMB manufactured by Tokuyama Corporation) is set in the central part of a reactor made of organic glass with a volume of 2000 mL, and the reactor is divided into two zones: a cathode zone and an anode zone.
[0205] In the cathode zone, add 100g of poorly soluble lead contaminated soil (the lead concentration is 5000mg / kg dry soil; sampling location: A paint factory), 800mL tap water, 50mL 1:1 hydrochloric acid, made of Teflon (registered trademark) The resultant stirring blade was stirred at a speed of 500 rpm to obtain the system under test.
[0206] Insert the cathode electrode of the copper metal mesh into the test system and connect it to the anode through a constant potential device (constant voltage power supply device).
[0207] In the anode zone, add 800 mL of tap water, 5 mL of 1:1 hydrochloric acid, and insert the grap...
Embodiment 2
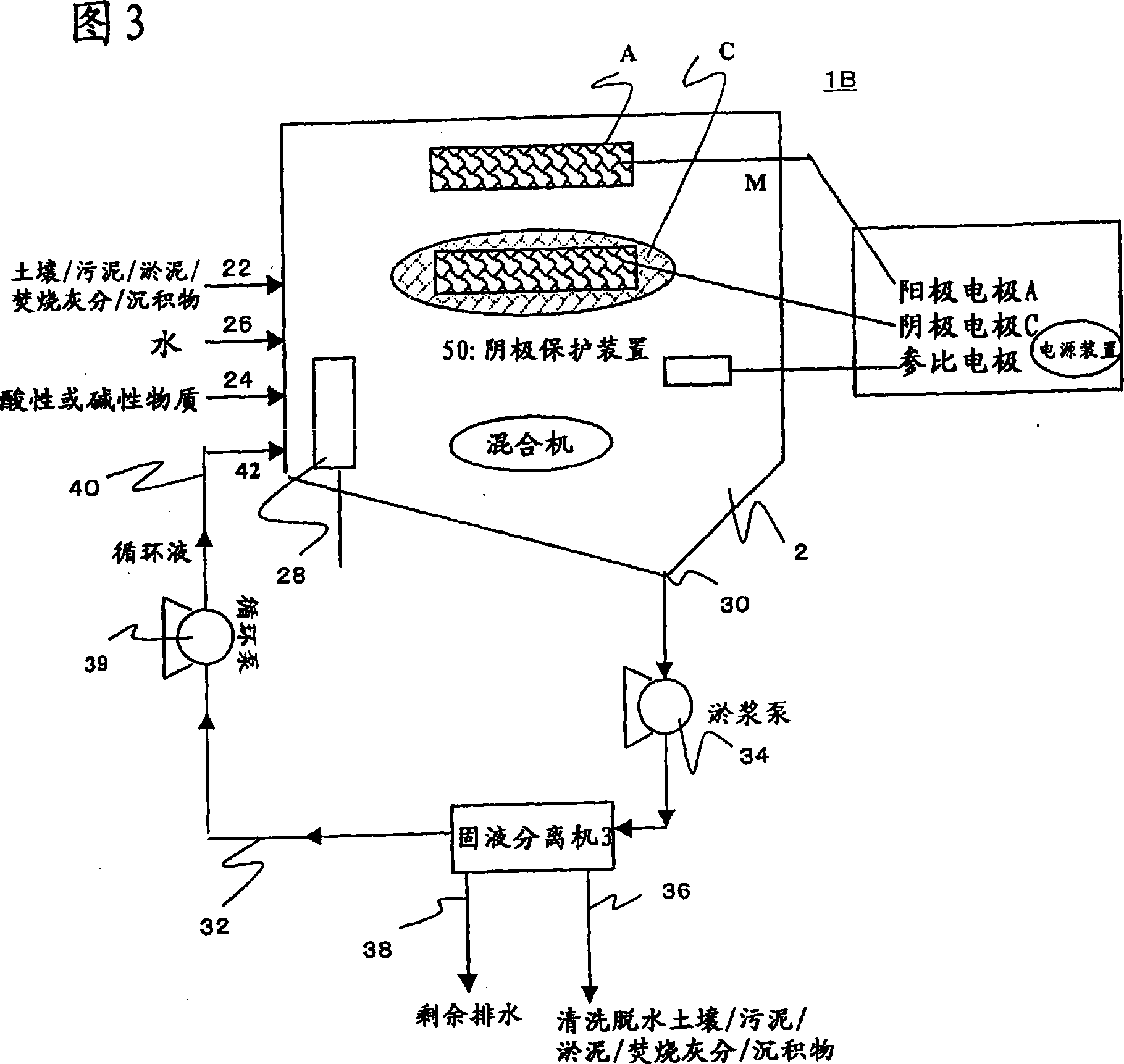
[0214] [Example 2] Electrode reduction and cleaning test of mercury contaminated sediment
[0215] As shown in Figure 2, a cation exchange membrane (NEOSEPTA CMB manufactured by Tokuyama Corporation) is installed in the central part of the reactor made of plexiglass with a volume of 2000 mL, and the reactor is divided into two zones: a cathode zone and an anode zone.
[0216] In the cathode zone, add 100g of insoluble lead contaminated sediment (total mercury concentration is 125mg / kg dry soil; sampling location: B pharmaceutical factory), 800mL tap water, 50mL 1:1 hydrochloric acid, using Teflon (registered trademark) ) The manufactured stirring blade was stirred at a speed of 500 rpm, and a nitrogen gas diffuser was inserted, and the cathode area was aerated with nitrogen at a speed of 10 mL / min as the system to be tested.
[0217] Insert the cathode electrode of the titanium metal mesh into the test system, and connect with the anode electrode through a potentiostat. In order t...
Embodiment 3
[0225] [Example 3] The influence of the diaphragm on the electrolysis removal of lead-contaminated soil
[0226] As shown in Figure 1, a cation exchange membrane (NEOSEPTA CMB manufactured by Tokuyama Corporation) is set in the central part of a reactor made of organic glass with a volume of 2000 mL, and the reactor is divided into two zones: a cathode zone and an anode zone. In the cathode zone, add 100g of poorly soluble lead contaminated soil (the lead concentration is 5000mg / kg dry soil; sampling location: A paint factory), 800mL tap water, 50mL 1:1 hydrochloric acid, made of Teflon (registered trademark) The resultant stirring blade was stirred at a speed of 500 rpm to obtain the system under test. Insert the cathode electrode of the copper metal mesh into the test system and connect it to the anode through a constant potential device (constant voltage power supply device). In the anode zone, 800 mL of tap water and 5 mL of 1:1 hydrochloric acid were added, and a titanium ano...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com