Antineoplastic active ferrum complex and method for preparing same
An anti-tumor activity, iron complex technology, applied in the direction of anti-tumor drugs, iron-organic compounds, drug combinations, etc., to achieve the effect of excellent liver cancer inhibition effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
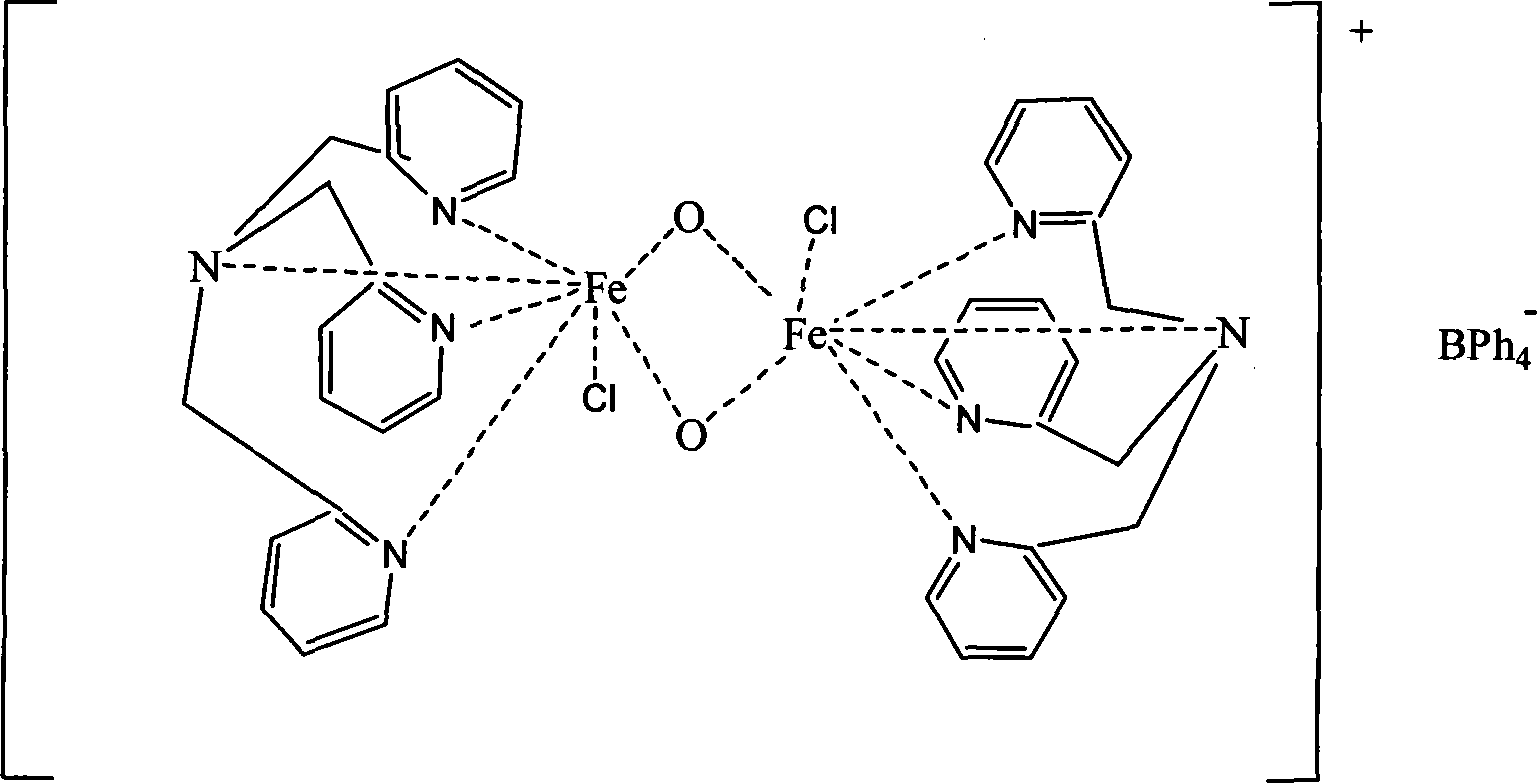
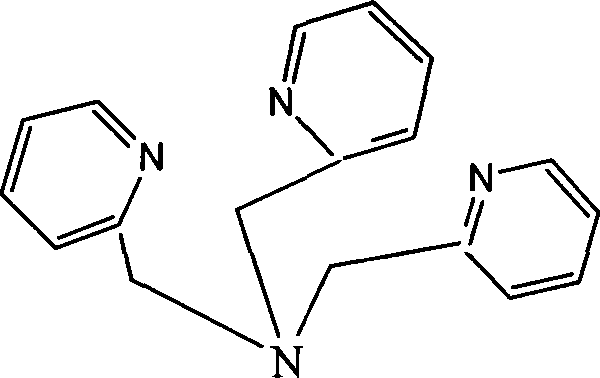
[0048] 1. Synthesis of iron complexes
[0049] Drop 5ml of acetonitrile solution containing 1mmol of 2-chloromethylpyridine into a round-bottomed flask containing 1mmol of dipyridylamine in 20ml of acetonitrile solution, and stir at room temperature for 3 hours; distill to dryness to obtain a yellow oil, and recover 20ml of acetonitrile . Then use 20ml CH 2 Cl 2 Extract, wash with 10ml of saturated sodium sulfate and 10ml of water, separate with a separatory funnel, and discard the aqueous layer. 20mlCH 2 Cl 2 The solution was placed in a 50ml Erlenmeyer flask, dried with anhydrous sodium sulfate, filtered, and the solvent was distilled off to obtain trippicolylamine (L) as a yellow oil.
[0050] The synthesized tripyridylamine was mixed with 1mmol FeCl 3 , 10mL methanol solution, stir at room temperature for 2 hours, add 1mmol NaBPh 4 A dark red precipitate was obtained. Filtrate, wash the precipitate with a small amount of water and ethanol, and dry in vacuum to obta...
Embodiment 3
[0053] 1. Synthesis of iron complexes
[0054] Drop 5ml of acetonitrile solution containing 1mmol of 2-chloromethylpyridine into a round-bottomed flask containing 1mmol of dipyridylamine in 20ml of acetonitrile solution, stir at 50°C for 2 hours; distill to dryness to obtain a yellow oil, recover 20ml Acetonitrile. Then use 20ml CH 2 Cl 2 Extract, wash with 10ml of saturated sodium sulfate and 10ml of water, separate with a separatory funnel, and discard the aqueous layer. 20mlCH 2 Cl 2 The solution was placed in a 50ml Erlenmeyer flask, dried with anhydrous sodium sulfate, filtered, and the solvent was distilled off to obtain trippicolylamine (L) as a yellow oil.
[0055] The synthesized tripyridylamine was mixed with 1mmol FeCl 3 , 10mL methanol solution, react at 60°C for 0.5 hours, add 1mmol NaBPh 4 A dark red precipitate was obtained. Filtrate, wash the precipitate with a small amount of water and ethanol, and dry in vacuum to obtain a dark red powder. The dark re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com