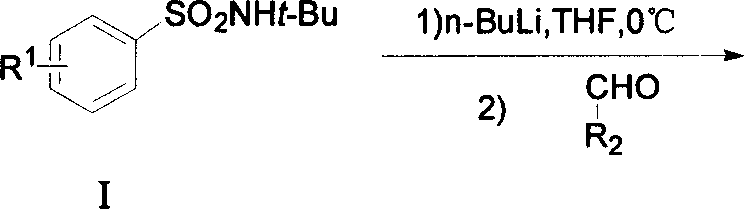Five-membered rings 3-position single-substituted benzosultam derivatives and preparation method thereof
A compound and aroyl technology, applied in the field of derivatives and their preparation, can solve the problems of low yield, expensive reagents, difficult preparation and the like, and achieve the effects of simple operation, good application prospect and good inhibitory activity of the preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1. Preparation of intermediate compound II
[0053] (1) Preparation of N-tert-butyl-2-[(4-chlorophenyl)-hydroxy-methyl]-benzenesulfonamide
[0054] Dissolve 2.13g (10mmol) of N-tert-butylbenzenesulfonamide intermediate in 30ml of dry tetrahydrofuran (THF), and slowly add 8.8ml of 2.5M n-butyllithium in n-hexane under ice bath After the addition of the solution, continue to stir for 20-30min, then slowly add 1.41g (10mmol) of p-chlorobenzaldehyde in THF. The reaction mixture was stirred for an additional 50-60 min. After TLC monitoring until the reaction is complete, add saturated NH 4 The Cl solution was fully stirred, and after extraction with ethyl acetate, the organic phase was washed with saturated brine, anhydrous Na 2 SO 4 dry. Separation and purification yielded white crystals with a yield of 90.6% and a melting point of 109-111°C.
[0055] Spectral analysis data: 1 HNMR (600MHz) 8.05 (dd, J = 7.9, 1.2Hz, 1H), 7.46 (dt, J = 7.6, 1.2Hz 1H), 7.38 ...
Embodiment 2
[0080] Embodiment 2. Preparation of target compound III
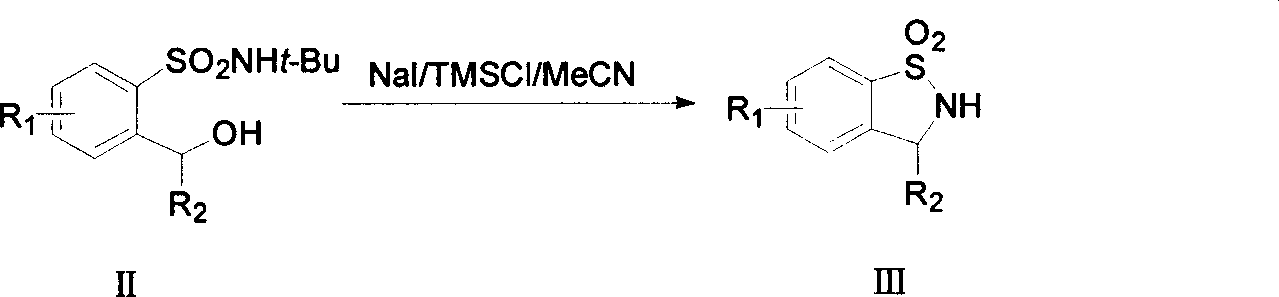
[0081] (1) Preparation of 3-(4-chlorophenyl)-1,1-dioxo-2,3-dihydro-benzo[d]isothiazole
[0082] Dissolve 0.36g (1mmol) of N-tert-butyl-2-[(4-chlorophenyl)-hydroxy-methyl]-benzenesulfonamide in 10ml of dry acetonitrile (CH 3 CN), add sodium iodide (NaI) 0.32g (2mmol), iodine (I 2 ) 0.13g (0.5mmol), then dropwise added 0.26ml (2mmol) of trimethylchlorosilane (TMSCl), after the addition was completed, the reaction was refluxed for 500-60min. After TLC monitors to react completely, add saturated Na 2 SO 3 The solution was fully stirred until the solution was colorless. After extraction with ethyl acetate, the organic phase was washed with saturated brine, anhydrous Na 2 SO 4 dry. Separation and purification yielded 0.25 g of white crystals with a yield of 89.6% and a melting point of 181-182°C.
[0083] Spectral analysis data: 1 HNMR (600MHz) 7.85(dd, J=5.8, 1.5Hz, 1H), 7.56-7.59(m, 2H), 7.37(dd, J=6.9, 1.1Hz, 2H), ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com