Synthesis method for alpha-carbonylenamine derivative cooling agent (3-MPC) and application thereof
A technology of ketoenamine and synthesis method, which is applied in the field of daily chemical products, can solve the problems of short coolness duration and strong eye irritation, and achieve the effects of obvious comfort, uniform coolness and long duration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
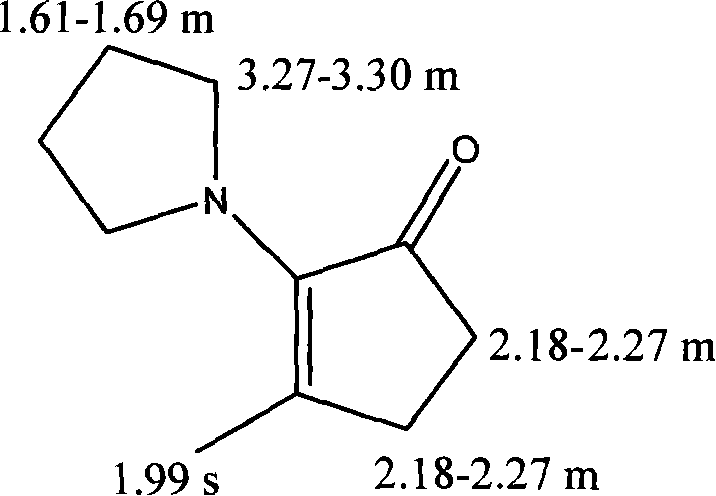
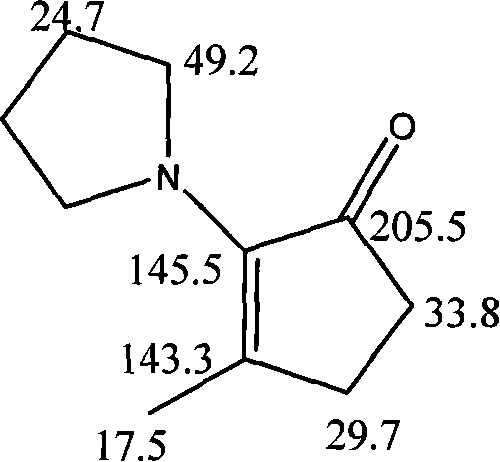
[0018] Take 33.6g of methylcyclopentenolone (MCP), 60.5g of tetrahydropyrrolidine, 0.19g of p-toluenesulfonic acid, (solvent 8 times the amount of the reaction material) 754g of methanol, and mix the methylcyclopentenolone with tetrahydropyrrolidine. Mix hydrogen pyrrolidine, add catalyst p-toluenesulfonic acid, quickly heat to reflux state, reflux time is 7 hours, stop the reaction and evaporate the solvent, add 283g water (3 times the amount of the reaction material), and then use 30% NaOH solution Adjust pH≈9, then extract the reaction solution with ether. After the extraction is complete, combine the organic layers, and then use 0.5mol / L Na for the organic layer 2 CO 3 Wash with solution, then use anhydrous Na 2 SO 4 After drying, use a rotary evaporator to evaporate the solvent to obtain a brownish-yellow oily liquid. Perform column chromatography with 400 mesh basic alumina. The eluent is petroleum ether: ethyl acetate=60:1 to obtain a light yellow oily liquid. α-ketoenamine...
Embodiment 2
[0025] Take 33.6g of methylcyclopentenolone (MCP), 63.8g of tetrahydropyrrolidine, 0.28g of p-toluenesulfonic acid, and 780g of methanol, mix methylcyclopentenolone and tetrahydropyrrolidine, add the catalyst pair Toluene sulfonic acid, quickly heated to reflux state, reflux time is 9 hours, stop the reaction and evaporate the solvent, add 293g of water, and then use 30% NaOH solution to adjust the pH≈9, and then extract the reaction solution with ether. After the extraction is complete , Combine the organic layer, and use 0.5mol / LNa for the organic layer 2 CO 3 Wash with solution, then use anhydrous Na 2 SO 4 Dry, use a rotary evaporator to evaporate the solvent to obtain a brownish-yellow oily liquid. Perform column chromatography with 400 mesh basic alumina. The eluent is petroleum ether: ethyl acetate=60:1, and a light yellow oily liquid is obtained. 3-MPC products. The product was characterized by infrared spectroscopy, mass spectrometry, and nuclear magnetic resonance spectr...
Embodiment 3
[0027] Take 33.6g of methylcyclopentenolone (MCP), 67.2g of tetrahydropyrrolidine, 0.5g of p-toluenesulfonic acid, and 810g of methanol, mix methylcyclopentenolone and tetrahydropyrrolidine, add the catalyst p-toluene Sulfonic acid, quickly heated to reflux state, reflux time is 11 hours, stop the reaction and evaporate the solvent, add 300g of water, and then use 30% NaOH solution to adjust the pH≈9, and then extract the reaction solution with ether. After the extraction is complete, Combine the organic layers, and then use 0.5mol / LNa for the organic layer 2 CO 3 Wash with solution, then use anhydrous Na 2 SO 4 After drying, use a rotary evaporator to evaporate the solvent to obtain a brownish-yellow oily liquid. Perform column chromatography with 400 mesh basic alumina. The eluent is petroleum ether: ethyl acetate=60:1, and a light yellow oily liquid is obtained. 3-MPC products. The product was characterized by infrared spectroscopy, mass spectrometry and nuclear magnetic resona...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com