Treatment for inflammation
A technology for a condition, arthritis, applied in the field of treatment of inflammatory diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
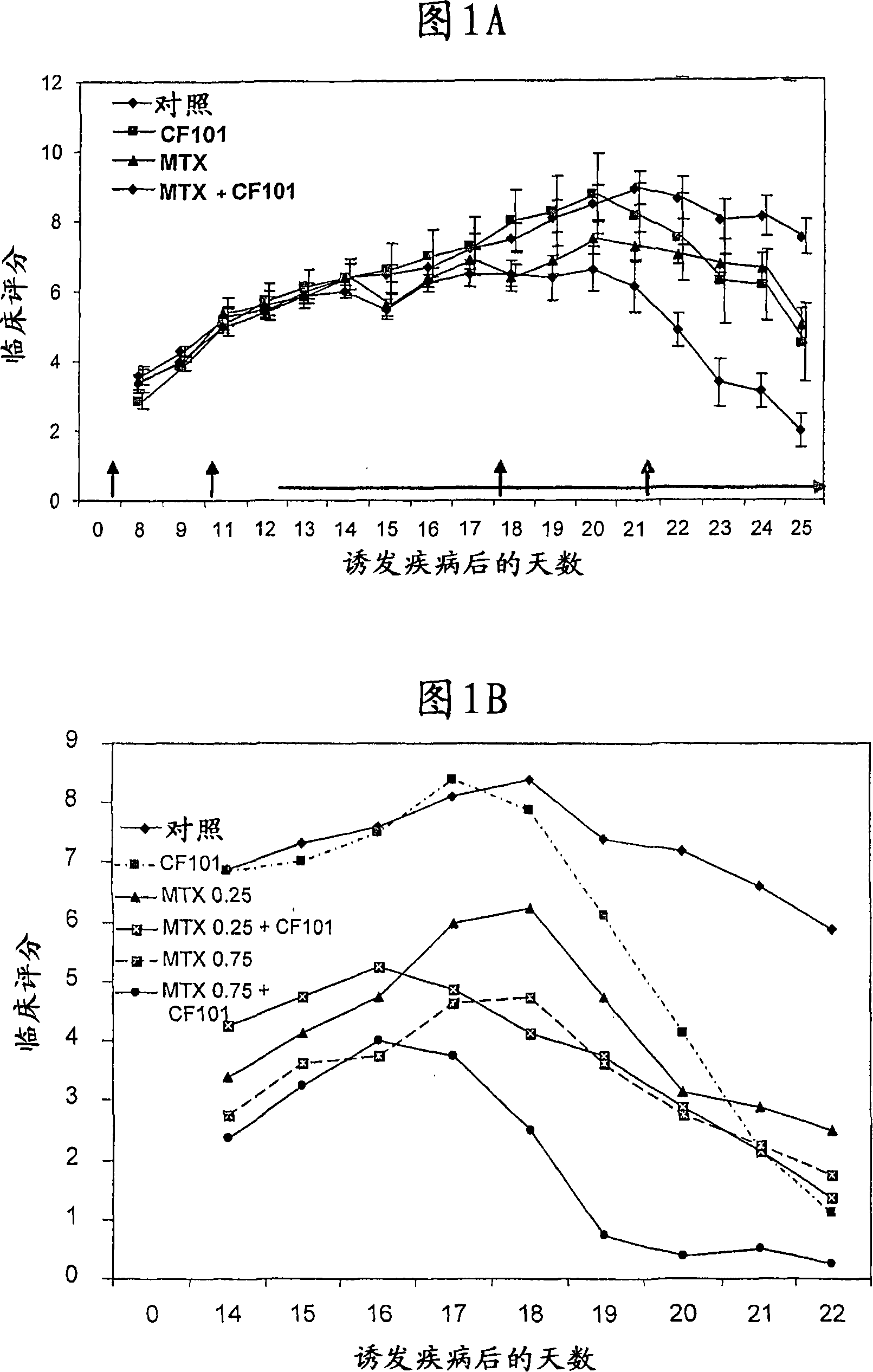
[0115] Example 1: In vivo studies
[0116] Material
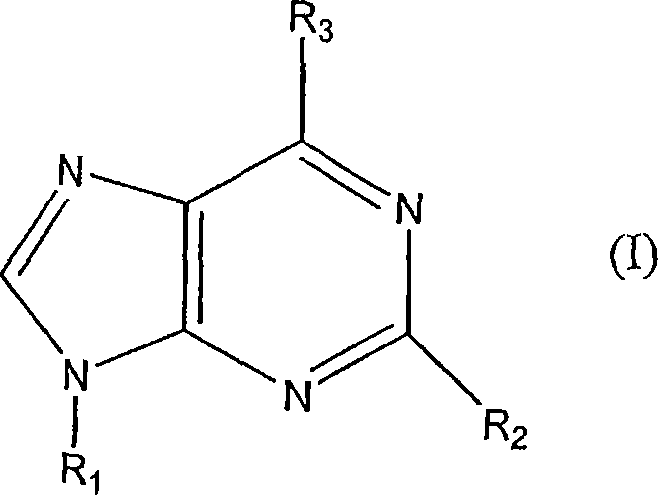
[0117] A was synthesized by Albany Molecular Research Inc. (Albany, NY, USA) 3AR agonist CF101, GMP grade compound, generically known as 1-deoxy-1-[6-[[(3-iodophenyl)methyl]amino]-9H-purin-9-yl]-N-methyl Base-D-ribofuranuramide (IB-MECA).
[0118] Methotrexate was purchased from Abie, Israel.
[0119] method
[0120] Female Lewis rats, 8-12 weeks old, were obtained from Harlan Laboratories (Jerusalem, Israel). Rats were fed standardized pelleted chow and provided with tap water. Experiments were performed in accordance with the guidelines established by the Institutional Animal Care and Use Committee of Can-Fite BioPharma, Petah Tikva, Israel. Rats were injected subcutaneously at the base of the tail with 100 μl of a suspension consisting of incomplete Freund's adjuvant (IFA) and 10 mg / ml heat-killed Mycobacterium tuberculosis (Mt) H37Ra (Difco, Detroit, USA). Each group contained 10 animals.
[0121] Treatment wi...
Embodiment 2
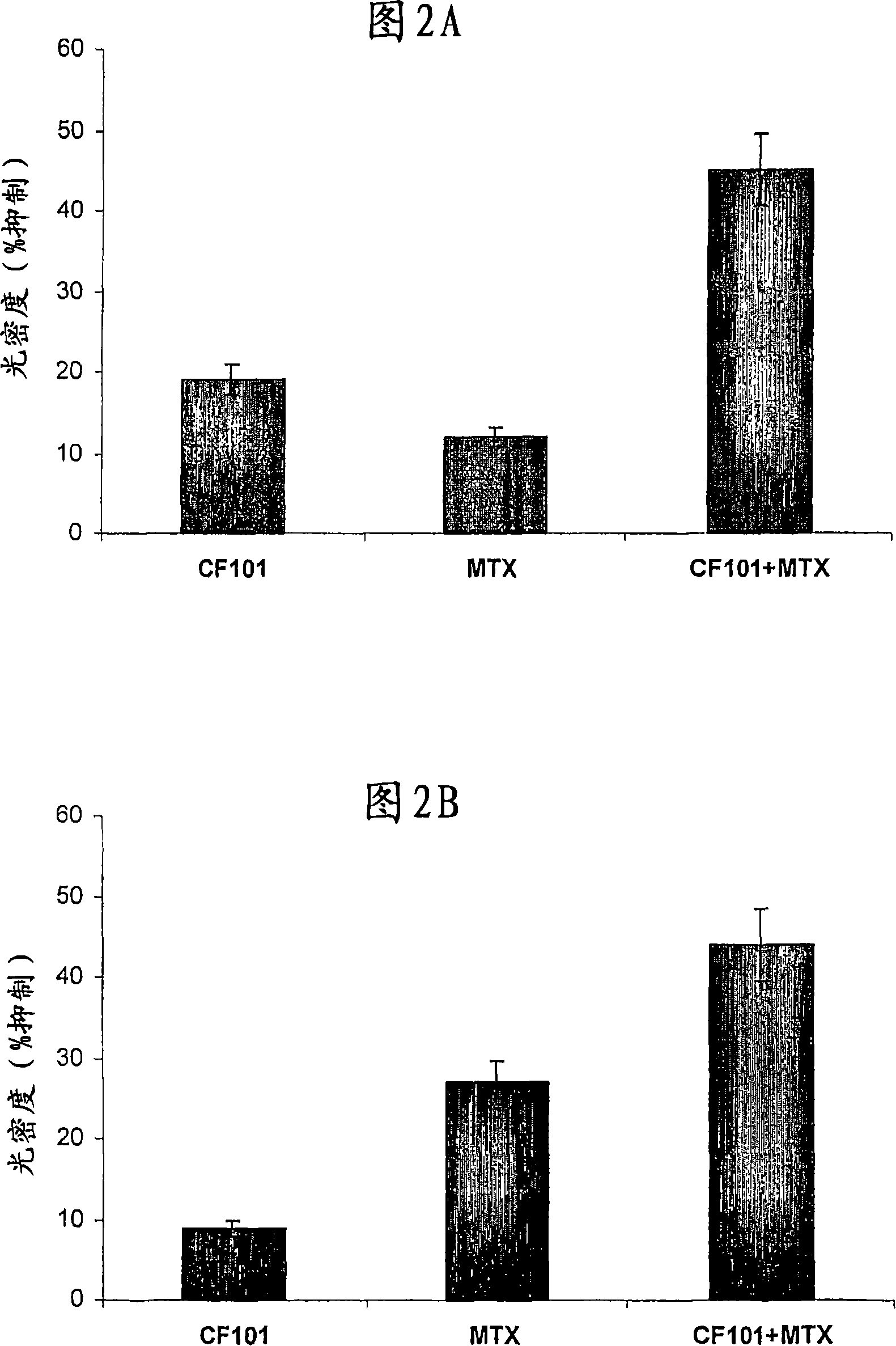
[0125] Example 2: In vitro studies
[0126] Culture of Human Fibroblast-Like Synoviocytes (FLS)
[0127] Human synovial fluid samples were collected from osteoarthritis (OA) patients undergoing paracentesis. The liquid was centrifuged and the supernatant was removed. Cells were resuspended in DMEM containing collagenase type I (4 mg / ml) for 2 hours with vigorous shaking at 37°C. Cells released in the supernatant were harvested by centrifugation and incubated at 37 °C, 5% CO 2 cultured in DMEM containing 10% FBS, 2mM glutamine, 100U / ml penicillin, 100μg / ml streptomycin, 1% non-essential amino acids, 1% sodium pyruvate and 20nM HEPES buffer. After overnight culture, non-adherent cells were removed. Adherent cells (FLS) were passaged at a 1:2 ratio, and cells from passages 4 to 10 were used in this experiment.
[0128] The effect of CF101 and methotrexate (MTX) together on the proliferation of FLS was examined using the MTT assay. Incubate cells in growth medium in 96-wel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com