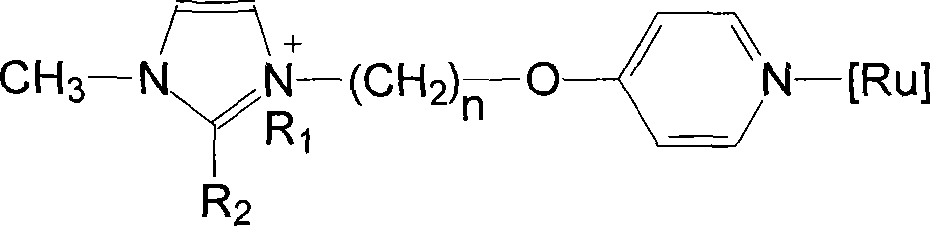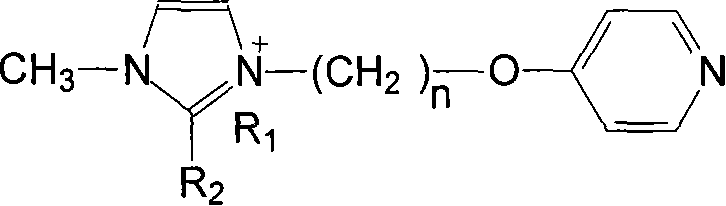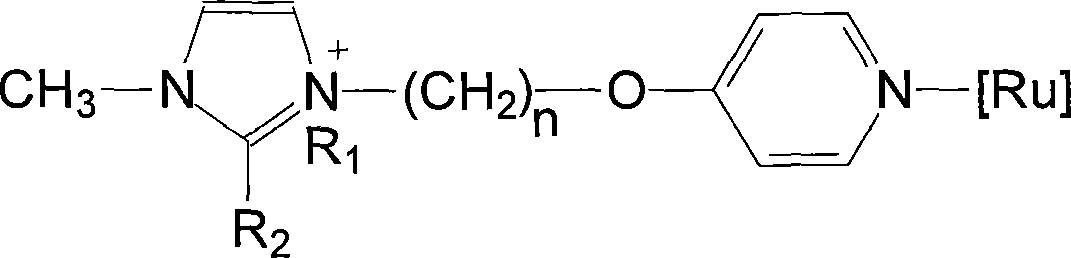Ionic liquid loaded ruthenium catalyst containing pyridine ligand and producing method thereof
A technology of ionic liquid and ruthenium catalyst, applied in the field of catalyst and its preparation, can solve problems such as poor solubility, and achieve the effect of improving poor solubility and expanding the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Synthesis of 1,2-Dimethyl-3-hydroxyethylimidazolium Chloride Ionic Liquid (1)
[0038]
[0039] Add 1,2-dimethylimidazole (19.2g, 200mmol) and chloroethanol (16.1ml, 240mmol) into a 250ml reaction flask, dissolve in 20ml of toluene, react at 110°C for 8 hours, stop the reaction, let it stand, and pour off the upper layer toluene. Then wash three times with toluene (20ml×3). Add 20ml of acetone to the crude product, stir at reflux at 70°C for 30min, let stand, and pour off the upper solvent. Repeat washing with acetone twice, let it stand, and cool down, and a light yellow solid is precipitated in the lower layer. The light yellow crude product was recrystallized from acetonitrile to obtain white crystals, which were dried in vacuo to obtain compound 1 (27.5 g, yield 78%). 1 H-NMR (D 2 O, δ): 7.35(d, 2H, N-CH=HC-N), 4.22(t, 2H, N-CH 2 ), 3.87(t, 2H, CH2 -OH), 3.74(s, 3H, N-CH 3 ), 2.56(s, 3H, C-CH 3 ); 13 C-NMR (D 2 O, δ): 146.36 (N-C-N), 123.76, 122.39 (HC=C...
Embodiment 2
[0041] Synthesis of 1,2-Dimethyl-3-hydroxyethylhexafluorophosphate Ionic Liquid (2)
[0042]
[0043] Will KPF 6 (13.2g, 72mmol) was dissolved in 80ml of water, cooled with an ice-water bath, and then a 50ml aqueous solution of compound 1 (10.6g, 60mmol) was slowly dropped into it. After the dropwise addition, the reaction was gradually raised to room temperature for 12 hours, and the reaction was stopped. The solvent water was removed to obtain a light yellow viscous liquid (containing white solid). Dissolved with 50ml of acetone, filtered, and the filtrate was dried with anhydrous sodium sulfate, and the solvent was removed to obtain a colorless viscous liquid, which was dried in vacuo to obtain compound 2 (14.2g, yield 83%). 1 H-NMR (D 2 O, δ): 7.40 (d, 2H, HC=CH), 4.27 (t, 2H, N-CH 2 ), 3.94(t, 2H, CH 2 -OH), 3.80(s, 3H, N-CH 3 ), 2.62 (s, 3H, C-CH 3 ); 13 C-NMR (D 2 O, δ): 146.36 (N-C-N), 123.76, 122.39 (HC=CH), 61.31 (CH 2 -OH), 51.53 (N-CH 2 ), 36.12 (N-CH...
Embodiment 3
[0045] Synthesis of 1,2-dimethyl-3-ethyl p-toluenesulfonate imidazolium ionic liquid (3)
[0046]
[0047] Under nitrogen protection, 2 (5.72g, 20mmol) was added to a 250ml Schlenk reaction flask, dissolved in 35ml of acetonitrile, then triethylamine (3.4ml, 24mmol) was added, cooled in an ice-water bath. p-Toluenesulfonyl chloride (4.58g, 24mmol) was dissolved in 25mL of acetonitrile and added dropwise under ice-bath conditions. After the dropwise addition, the reaction was maintained at 0-5°C for 1 hour, then raised to room temperature for 10 hours, and the reaction was stopped. Filter, remove solvent, obtain solid and dark viscous liquid, use 100ml CH 2 Cl 2 dissolved and washed several times with water, the organic phase was washed with anhydrous Na 2 SO 4 dry. Remove CH 2 Cl 2 A brown-yellow solid was obtained, which was recrystallized from acetone:ether=2:1 to obtain a light-yellow solid, which was dried in vacuo to obtain 4.84 g of the product, with a yield of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com