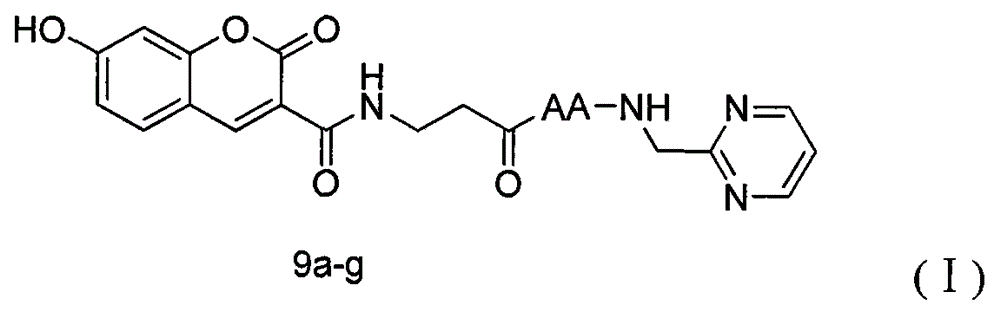
Coumarin derivative and preparation method thereof
A technology of hydroxycoumarin and compounds, applied in the field of biomedicine, can solve problems such as increasing myocardial infarction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
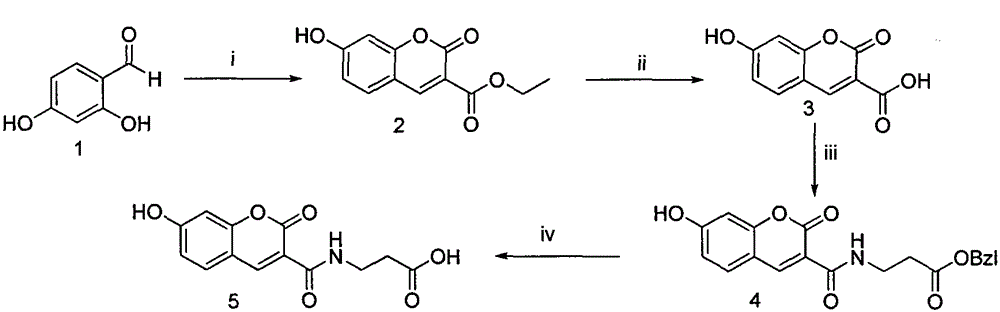
[0040] Embodiment 1 prepares 7-hydroxycoumarin-3-formic acid ethyl ester (2)
[0041] At a temperature of 90°C, weigh 2,4-dihydroxybenzaldehyde (0.500g, 3.60mmol) and measure diethyl malonate (0.70ml, 4.00mmol) into a 100ml eggplant bottle, dissolve in 95% In ethanol (20ml), the color of the solution was a reddish-brown transparent liquid at this time; piperidine (0.40ml, 4.00mmol) was added, the surface of the solution glowed green, and it began to reflux. After about 6 hours of reaction, the solution turned dark brown. Cooled to room temperature, concentrated under reduced pressure to red viscous, recrystallized from ethanol, and filtered to obtain compound 3 (0.758 g, 3.24 mmol), which was a light yellow solid with a yield of 90%. ESI-MS m / z 233 (M-1).
Embodiment 2
[0042] Embodiment 2 prepares 7-hydroxycoumarin-3-carboxylic acid (3)
[0043] At a temperature of 90°C, compound 3 (2.00g, 8.55mmol) was weighed and dissolved in water (20ml), concentrated hydrochloric acid (6ml) was added, and then refluxed for 8h. The reaction solution was cooled to room temperature, filtered and washed with water to obtain compound 4 (1.32 g, 6.41 mmol) as a light red solid with a yield of 75%. ESI-MS m / z 205 (M-1).
Embodiment 3
[0044] Example 3 Preparation of 7-hydroxycoumarin-3-formyl-β-propionic acid benzyl ester (4)
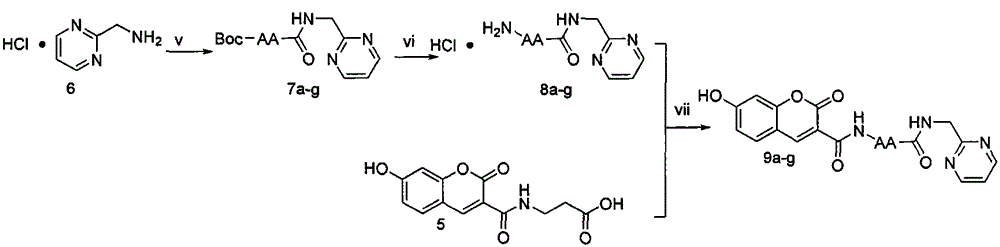
[0045] Weigh 3 (1.00g, 4.85mmol) and put it in a 100ml eggplant bottle, add tetrahydrofuran (30ml) under ice-bath cooling condition, add N-hydroxybenzotriazole (HOBt) (0.720g, 5.33mmol) successively, 1- (3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC·HCl) (1.02 g, 5.33 mmol), reacted for 30 minutes. Compound Tos·β-alanine-OBzl (1.87g, 5.33mmol) dissolved in tetrahydrofuran (20ml) was added, and N-methylmorpholine (NMM) (0.60ml) was added dropwise to adjust the pH to 8. TLC detection was carried out throughout the reaction, and the reaction was complete in about 8 hours. Filter, concentrate to dryness under reduced pressure, add ethyl acetate (100ml) to dissolve the residue, add the solution to a 250ml separatory funnel, wash with saturated sodium bicarbonate aqueous solution (50mL×3), saturated sodium chloride aqueous solution Wash (50mL×3), 5% potassium bisulfate aq...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com