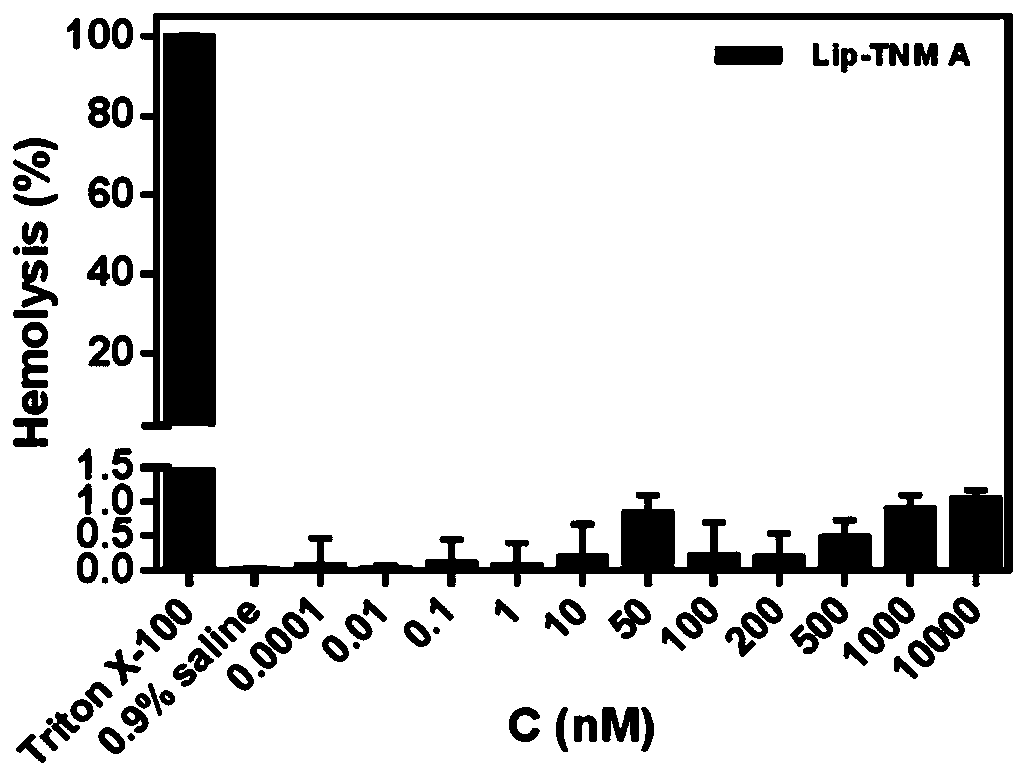
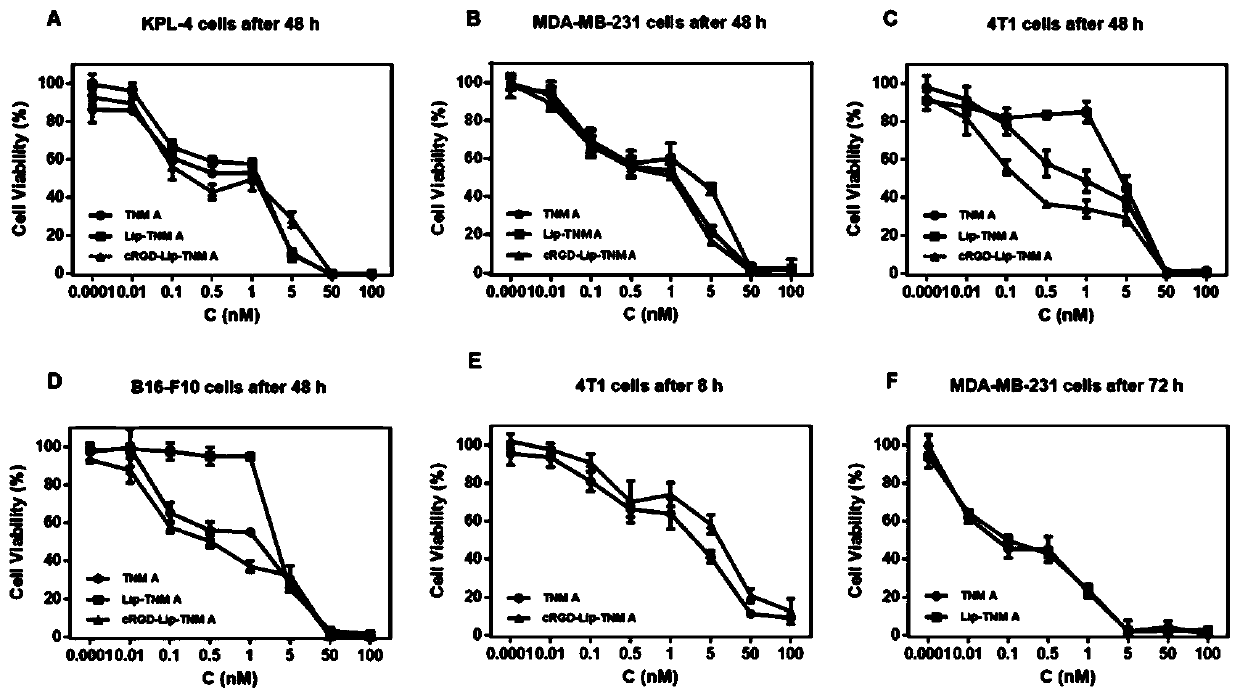
Tiancimycin lipidosome as well as preparation method and application of Tiancimycin lipidosome
A technology of tiancimycin lipid and tiancimycin, which is applied in the application field of liposome and liposome anti-tumor in vivo, can solve the problems of multi-drug resistance, achieve significant targeting, improve poor solubility, The effect of simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Preparation of Tiancimycin Liposome
[0052] Weigh 30 mg of soybean lecithin, 14 mg of hydrogenated soybean lecithin, 15 mg of cholesterol, 10 mg of DSPE-PEG2000, 400 μL of TNMA (methanol mother liquor 1 mg / mL) and dissolve in 5 mL of dichloromethane, place in a rotary evaporating flask, and decompress Uniform film formation by rotary evaporation. Take another 5 mL of PBS aqueous solution, put it in the round-bottomed flask where the film has been formed, rotate to hydrate the film, disperse it ultrasonically in an ice-water bath for 15 min, and centrifuge it in an ultrafiltration centrifuge tube (MW 3500) (4000 rpm) for 15 min. An opalescent liposome solution was obtained. The particle size of the liposome liposome was measured to be 93 nm, and the Zeta potential was -4.5 mV.
Embodiment 2
[0054] Preparation of liposomes targeting Tiancimycin
[0055] Weigh and weigh 30 mg of soybean lecithin, 14 mg of hydrogenated soybean lecithin, 15 mg of cholesterol, 10 mg of DSPE-PEG2000, 6 mg of DSPE-PEG-cRGD, 400 μL of TNM A (methanol mother solution 1 mg / mL) and dissolve in 5 mL of dichloromethane placed in a rotary evaporating flask, and evaporating under reduced pressure to form a uniform film. Take another 5 mL of PBS aqueous solution, put it in the round-bottomed flask where the film has been formed, spin to hydrate the film, disperse it ultrasonically in an ice-water bath for 15 min, and centrifuge it in an ultrafiltration centrifuge tube (MW 3500) (4000 rpm) for 15 min to obtain Opalescent liposome solution. The particle size of liposomes targeted to Tiancimycin was measured to be 99 nm, and the Zeta potential was -5.1 mV.
Embodiment 3
[0057] In Vitro Release Test of Tiancimycin Liposome
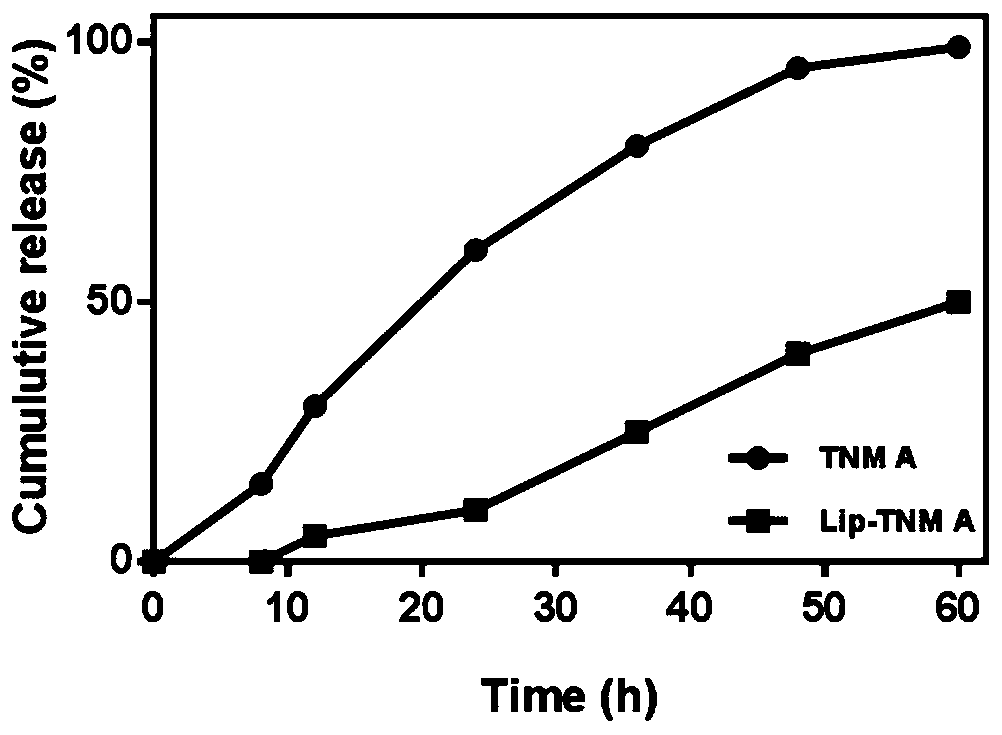
[0058] Experimental method: cut small pieces of dialysis bags (MW 3500), boil them in boiling water for 10 minutes, take them out and soak them in pure aqueous solution, take 1.0 ml each of the orthomycin solution and the orthomycin liposome solution, and put them into the pretreated The two ends of the dialysis bag were tied tightly, and the dialysis bag was placed in 20 mL of PBS buffer solution (0.5% SDS) and shaken in a 37°C constant temperature shaker at 200 r / min. Take 1ml of each sample at the designated time points, and detect the content of orthomycin by HPLC. Add 1 ml to the dialysate after each sampling. The results of in vitro release experiments were as follows: figure 1 As shown, the results showed that: the cumulative release of free tiaciline reached 99% in the first 60 h, and the release of tibialcin liposomes (Lip-TNM A) under physiological conditions (pH 7.4, 37°C, 0.5% SDS) No burst release was shown...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com