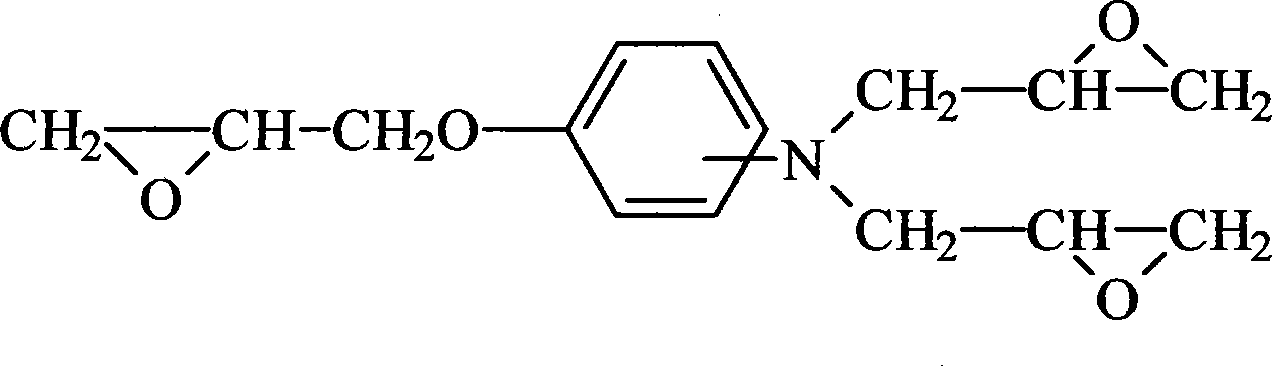
Method for preparing aminophenol triglycidyl group compound
A technology of triglycidol and aminophenol, applied in the direction of organic chemistry, can solve the problems of shortening reaction time and increasing cost, and achieve the effect of shortening reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Put 1203g (13 moles) of epichlorohydrin and 552g of ethylene glycol, blown nitrogen, stirred and added 109g (1 mole) of p-aminophenol in batches at 40°C, controlled to complete the addition within 2.5 hours, and kept the temperature for 4 hours when the temperature rose to 50°C. Then, 320 g (4 moles of NaOH) of 50% by weight sodium hydroxide aqueous solution was added dropwise, and added dropwise into the reaction system three times, and reacted at 55° C. for 3 hours after the dropwise addition was completed. Followed by vacuum distillation, recovery of epichlorohydrin, distillation at 60°C / 600mmHg until there is almost no distillate. The distillate was allowed to stand overnight and separated into layers, epichlorohydrin was circulated as the raw material for the reaction, the mixture of water and ethylene glycol was excluded, and another treatment was performed. After epichlorohydrin was distilled under reduced pressure, the residue was extracted with 1200ml of tolue...
Embodiment 2
[0019] In addition to replacing p-aminophenol with m-aminophenol, and replacing ethylene glycol with ethanol, the amount of epichlorohydrin added is 1388g (15 moles), the feeding temperature is 25°C, the feeding time is 3 hours, and the temperature is kept at 55°C for 5 hours. After the alkali is finished, react at 60° C. for 3 hours, distill out epichlorohydrin at a temperature of 65° C., and a pressure of 500 mmHg. Other formulations and operating steps are exactly the same as in Example 1. As a result, a yellow-brown m-aminophenol triglycidyl compound was obtained, with a yield of 263 g, a yield of 95%, an epoxy value of 0.85, a viscosity of 2490 mPa.S, and a Gardner hue of 5.
Embodiment 3
[0021] In addition to replacing p-aminophenol with o-aminophenol and isopropanol instead of ethylene glycol, the amount of epichlorohydrin added is 833g (9 moles), the feeding temperature is 55°C, the feeding time is 2 hours, and it is kept at 45°C for 3 hours. Hour, other formula and operating procedure are exactly the same as embodiment 1, the result obtains yellow-brown o-aminophenol triglycidyl compound, and its yield is 269g, yield 97%, epoxy value 1.00, viscosity 2485mPa.S, Gardner hue is 7.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com