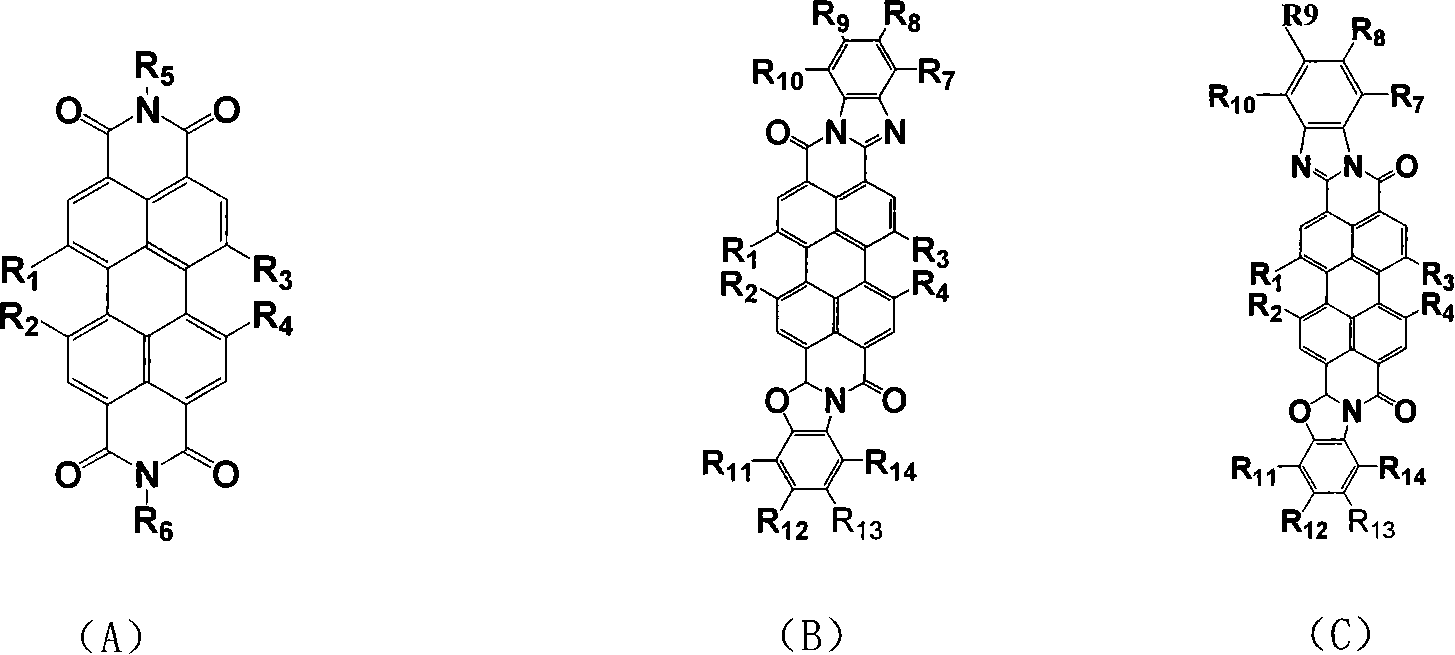
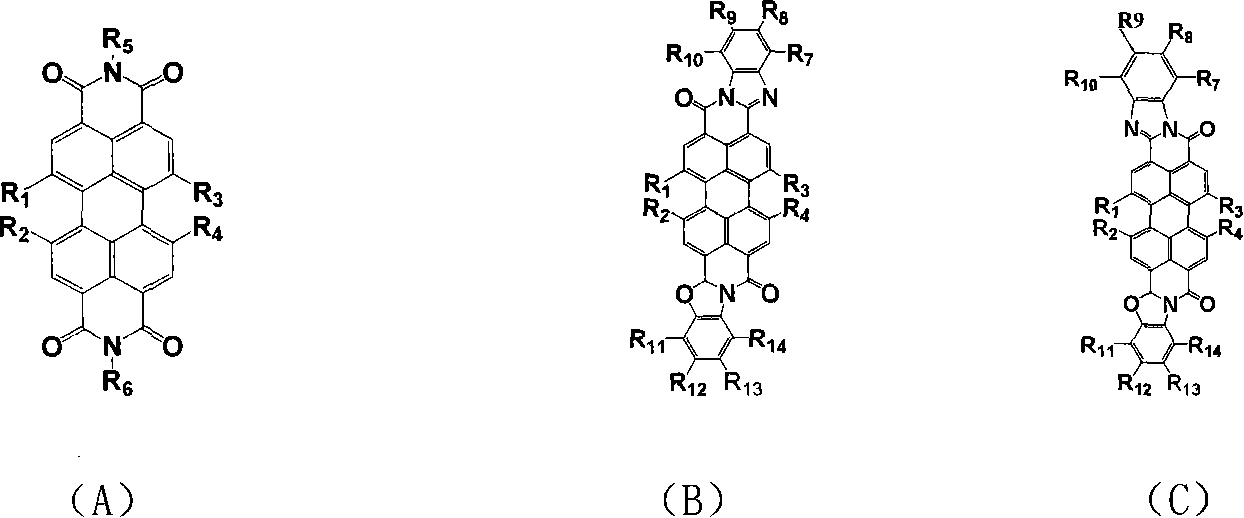
Perfluoro alkyl substituted perylene bis diimines and derivative and preparation method thereof
A perylene bis-diimide and perfluoroalkyl technology, which is applied in the fields of organic and fine chemicals, can solve the problems that the solubility of perylene bis-diimide or bisbenzimidazole perylene cannot be significantly increased, and achieve increased stability, Ease of operation and improved solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1 Preparation of 1-perfluorooctyl N, N'-diisooctyl perylene bis-diimide
[0019]
[0020] In a 5mL flask, add 49mg of 1-bromo-N,N'-diisooctylperylenebisdiimide (0.071mmol), 65mg of copper powder (1.0mmol), 1mL of N-methylpyrrolidone, 120μL (0.40mmol) of iodoperylene Fluorooctane, under the protection of Ar, kept the system at 100°C, reacted for 13h, and passed through a silica gel column to obtain 41 mg of 1-perfluorooctyl N,N'-diisooctylperylene bis-diimide, melting point 178°C, yield 50 %, MS: 1032; 1 H-NMR (400M, CDCl 3 ): δ=0.85-1.98(m, 30H), 4.19(m, 4H, CH 2 ), 8.34 (d, 1H, perylene ring), 8.64 (m, 3H, perylene ring), 8.75 (d, 1H, perylene ring), 8.80 (d, 1H, perylene ring), 8.93 (s, 1H, perylene ring ); 13 C-NMR (400M, CDCl 3 ):δ=10.79,14.26,23.25,24.23,28.88,30.95,38.18,44.55,122.68,123.00,123.91,124.20,124.67,126.70,128.36,129.48,130.27,131.21,132.04,132.26,132.67,133.10,135.19, 137.71, 163.03, 163.52, 163.58, 163.82; 19 F-NMR (400M, CDCl 3 ):...
Embodiment 2
[0021] Example 2 Preparation of 1,7-diperfluorooctyl N, N'-dioctylperylenebisdiimide
[0022]
[0023] Add 83mg (0.11mmol) 1,7-dibromo-N,N'-dioctylperylene bis-diimide, 66mg (1.0mmol) copper powder, 1mL N-methylpyrrolidone, 150μL (0.56mmol) iodine to a 5mL flask Substituted perfluorooctane, under the protection of Ar, kept the system at 80°C, reacted for 12h, and passed through a silica gel column to obtain 41mg of 1,7-diperfluorooctyl N,N'-dioctylperylene bis-diimide, melting point 105°C , yield 25.9%, MS: 1450.
Embodiment 3
[0024] Example 3 Preparation of 1-perfluorohexyl N, N'-dicyclohexyl perylene bis-diimide
[0025]
[0026] Add 64mg 1-bromo-N,N'-dicyclohexylperylenebisdiimide (0.10mmol), 64mg copper powder (1.0mmol), 1mL N-methylpyrrolidone, 180μL (0.40mmol) Fluorohexane, under the protection of Ar, keep the system at 100°C, react for 24h, and pass through a silica gel column to obtain 45 mg of 1-perfluorohexyl N,N'-diisooctylperylene bis-diimide, melting point 164°C, yield 52% .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com