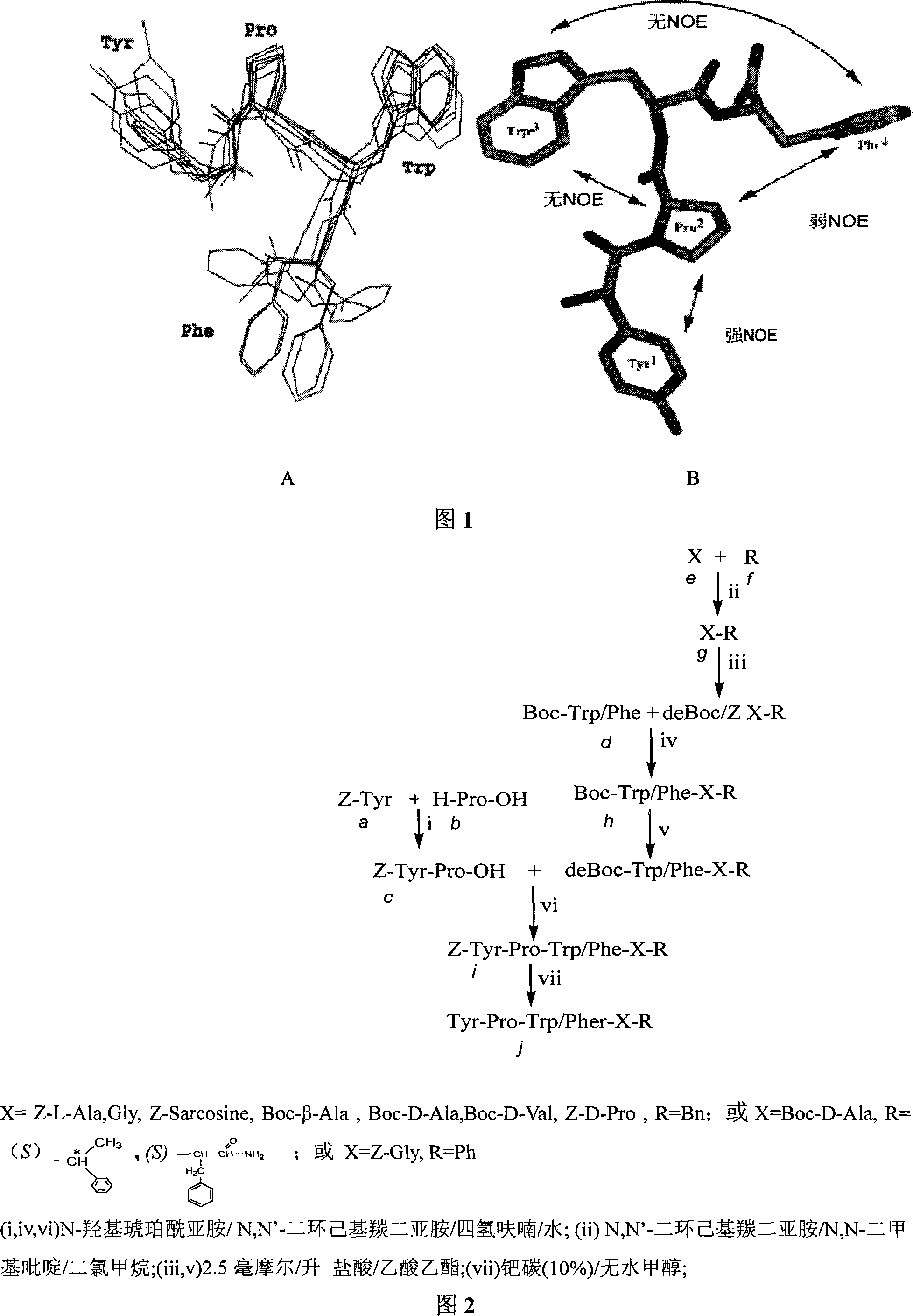
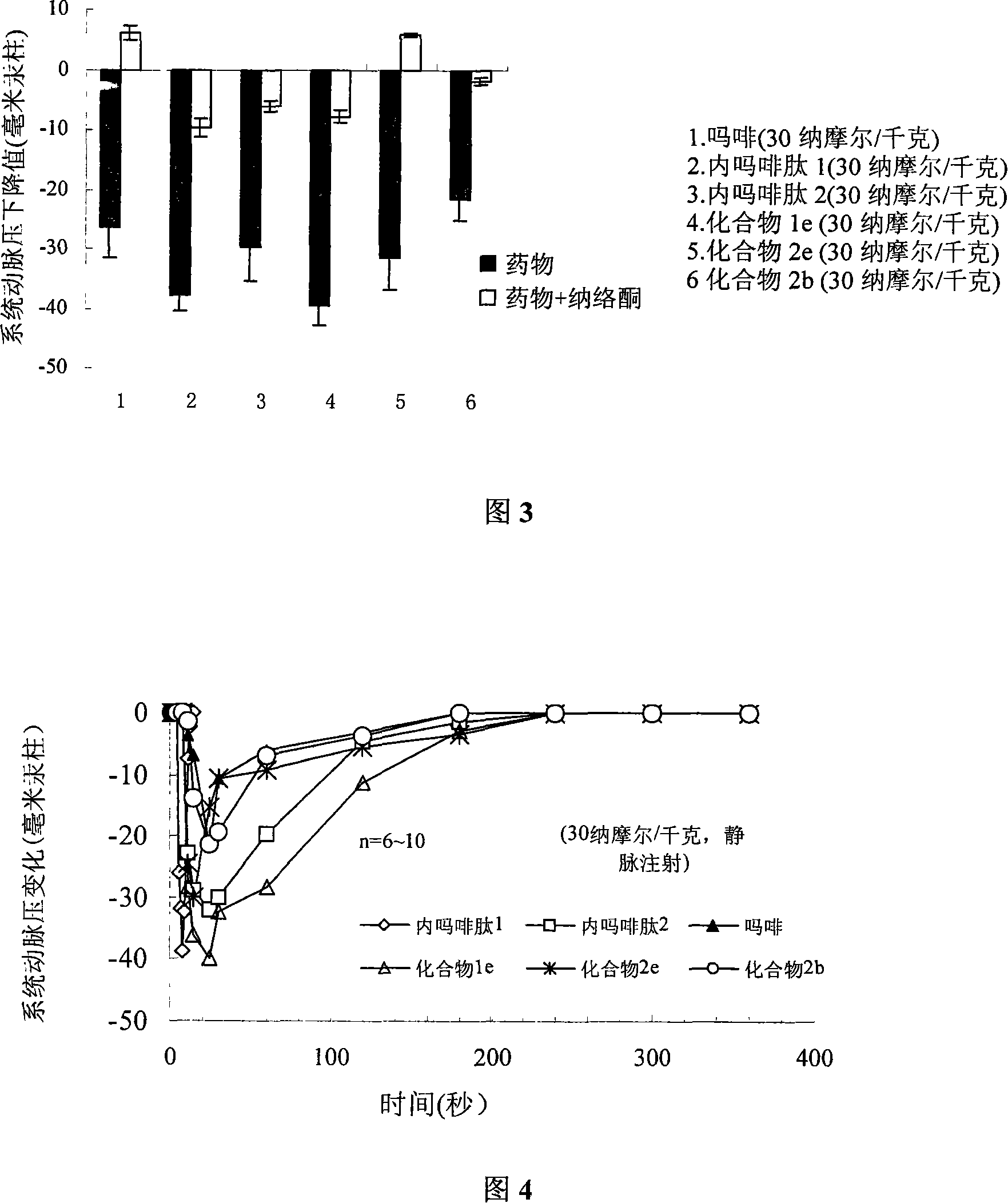
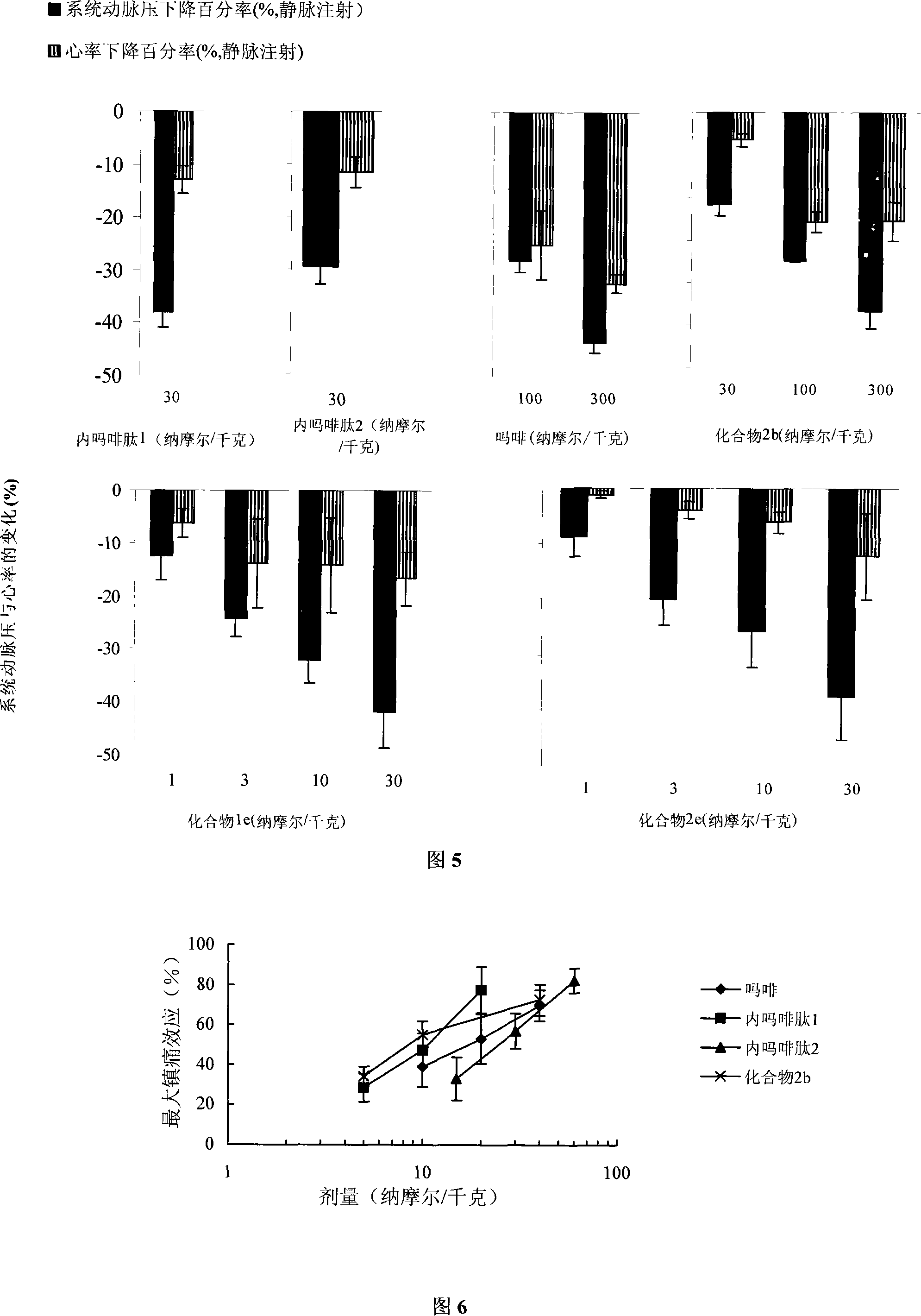
C end modification endomorphin 2
An endomorphin and structural feature technology, applied in the field of physiological activity research, can solve the problems of difficult to break through the blood-brain barrier, unfavorable for oral administration and injection, etc., and achieve the effects of low cost, high yield and reduced workload
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0036] Example of Synthesis: Synthesis of Compound 1e (Tyr-Pro-Trp-D-Ala-NH-Bn)
[0037] Reaction i: Synthesis of benzyloxycarbonyl-tyrosine-proline (c): Compound a 0.946g (3mmol), Hosu 0.346g (3.3mmol) were dissolved in anhydrous tetrahydrofuran (THF), stirred in ice bath for 10 minutes 0.619 g (3.6 mmol) of DCC were added afterwards. After reacting in ice bath for 30 minutes, remove the ice bath, react at room temperature for 3 to 5 hours, filter with Buchner funnel, remove the DCU (cyclodihexyl urea) generated by the reaction to obtain the activated ester THF solution of compound a. 0.414g (3.6mmol) of compound b was dissolved in equimolar sodium hydroxide aqueous solution, and the pH value was adjusted to 9-10. After stirring in an ice bath for 10 minutes, THF solution of the activated ester of compound a was added. After reacting in an ice bath for 30 minutes, remove the ice bath, and react overnight at room temperature. Drain THF, dissolve in ethyl acetate, wash with 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com