Potentiator for radiation therapy comprising pyridine derivative as active ingredient
A technology of radiation and derivatives, applied in the direction of active ingredients of heterocyclic compounds, medical preparations containing active ingredients, organic chemistry, etc., can solve problems such as development problems, and achieve the effect of reducing side effects and excellent cancer treatment effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
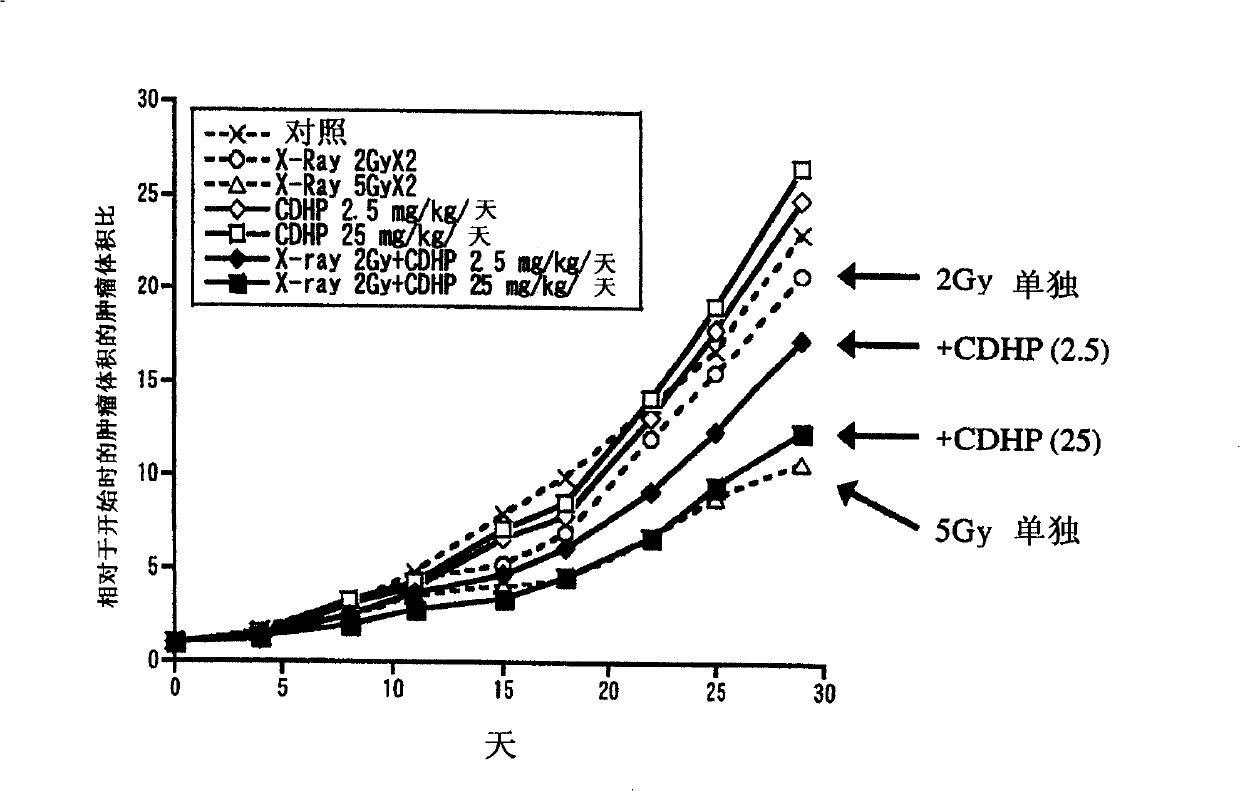
Examples
preparation example 1
[0080] Preparation example 1 tablet
[0081] CDHP 18mg
[0082] Starch 110mg
[0083] Magnesium Stearate 17mg
[0084] Lactose 40mg
[0085] Total 185mg
preparation example 2
[0086] With the above-mentioned compounding ratio, prepare 185 mg per tablet according to the usual method. Preparation example 2 tablet
[0087] CNDP 12mg
[0088] Lactose 54mg
[0089] Crystalline Cellulose 20mg
[0091] Talc 3mg
[0092] Methylcellulose 10mg
[0093] Total 104mg
[0094] With the above compounding ratio, according to the usual method, prepare each 104mg tablet formulation example 3 granules
[0095] CDHP 58mg
[0096] Lactose 340mg
[0097] Corn starch 450mg
[0098] Hydroxypropyl Methyl Cellulose 10mg
[0099] Total 858mg
[0100] Granules are prepared according to the usual method at the above-mentioned compounding ratio.
[0101] Preparation example 4 suppositories
[0102] CDHP 110mg
[0103] Witepsol W-35 900mg
[0104] Total 1010mg
[0105] With the above-mentioned compounding ratio, suppositories are prepared according to the usual method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com