Nanoparticulate formulations of docetaxel and analogues thereof
A docetaxel and nanoparticle technology, which is applied in the fields of prostate cancer, lung cancer, ovarian cancer, and breast cancer treatment, can solve the problem of no description of docetaxel nanoparticle preparation, etc., and achieve the effect of rapid drug dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0160] The preparation of povidone polymers results in polymers containing molecules of unequal chain length, and therefore of different molecular weights. The average molecular weight for each particular commercially available grade varies. Because it is difficult to directly determine the molecular weight of polymers, the most widely used method for classifying various molecular weights is the K-value method based on viscosity measurements. The K values for the various grades of povidone polymers have a function representing the average molecular weight, which is obtained from viscosity measurements and calculated according to Fikentscher's formula.
[0161] The average weight (Mw) of molecular weights is determined by methods that measure the weight of individual molecules, such as light scattering. Table 1 provides molecular weight data for several commercially available povidone polymers, all of which are soluble.
[0162]While applicants do not wish to be bound by a ...
Embodiment 1
[0260] The purpose of this example was to prepare a nanoparticulate anhydrous docetaxel formulation.
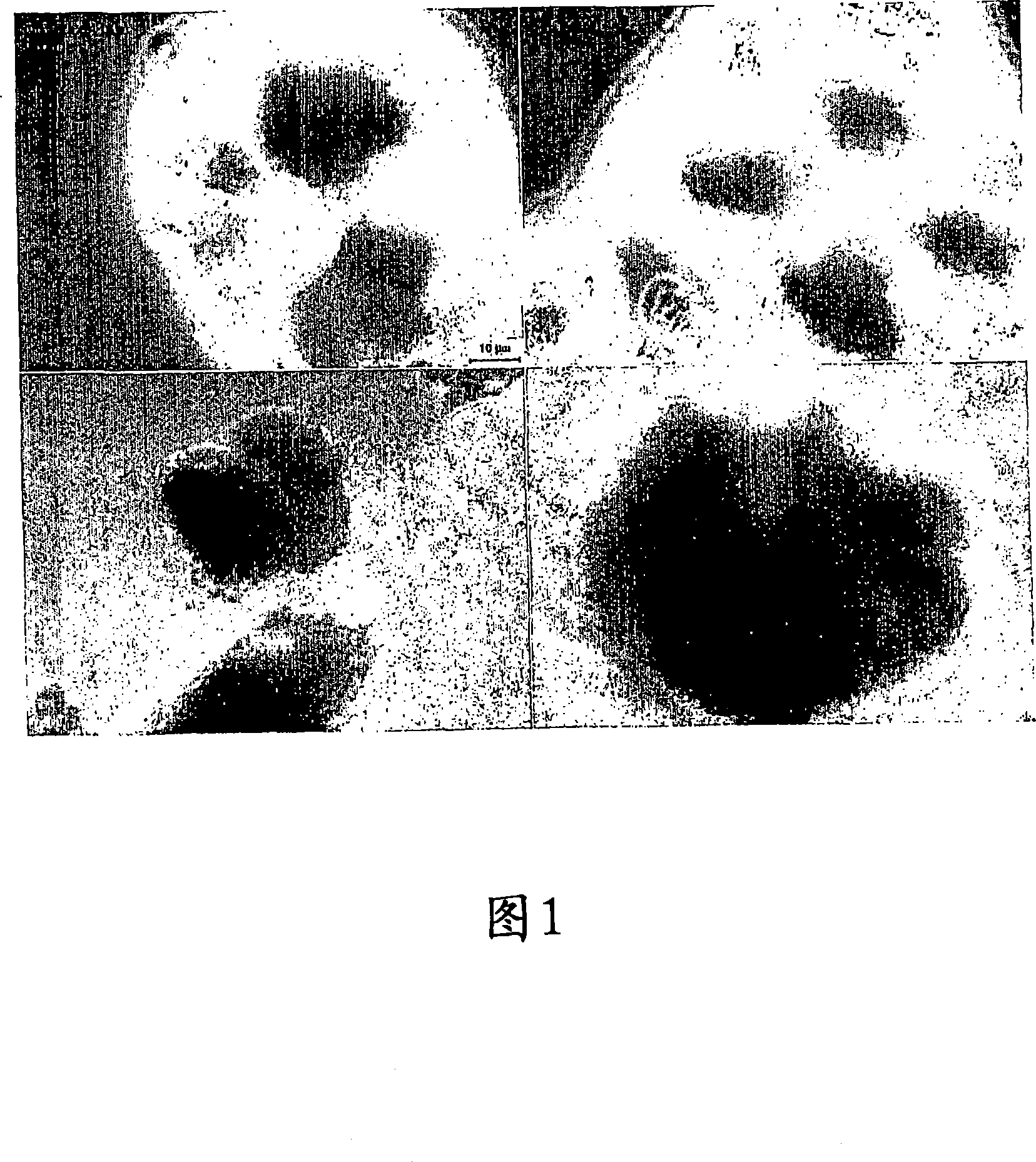
[0261] Figure 1 shows a light micrograph of unmilled Docetaxel (anhydrous) (Camida Ltd.), showing that conventional non-nanoparticulate Docetaxel (anhydrous) has an average particle size of 212,060 nm, D50 is 175,530nm and D90 is 435,810nm.
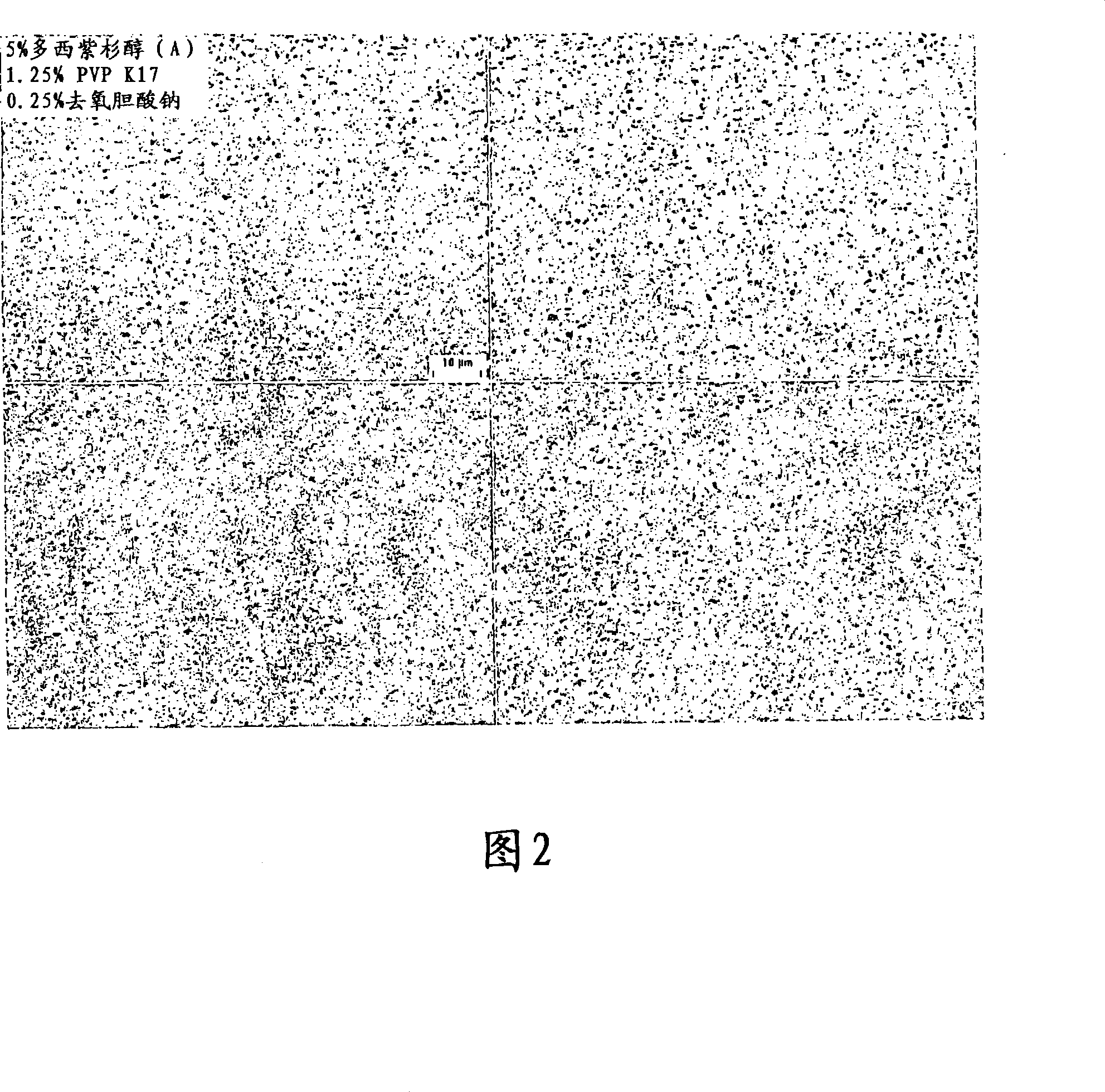
[0262] Aqueous dispersion of 5% (w / w) Docetaxel (Camida Ltd.) with 1.25% (w / w) polyvinylpyrrolidone (PVP) K17 and 0.25% (w / w) sodium deoxycholate merge. Then to NanoMill _ 0.01 of this mixture was added to a 10 ml chamber (NanoMill Systems, King of Prussia, PA; see US Pat. No. 6,431,478), along with a 220 micron PolyMill _ Grinding media (Dow Chemical) (89% media loading). The mixture was milled at 2500 rpm for 180 minutes.
[0263] After milling, the particle size of the milled docetaxel particles was then measured in deionized distilled water using a Horiba LA 910 particle size analyzer. The mean particle size of the milled docet...
Embodiment 2
[0266] The purpose of this example was to prepare a nanoparticulate anhydrous docetaxel formulation.
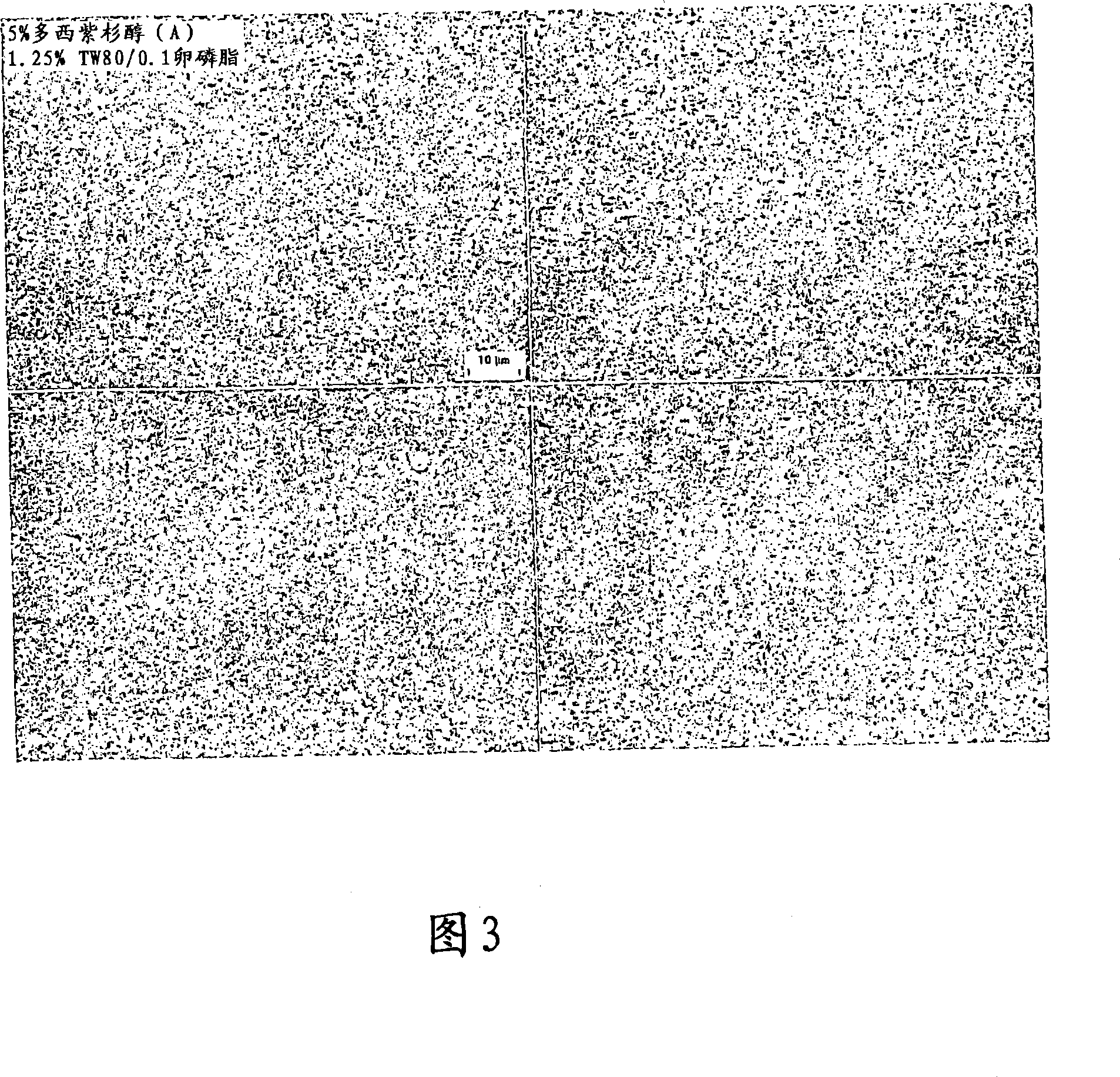
[0267] Aqueous dispersion of 5% (w / w) anhydrous docetaxel with 1.25% (w / w) Tween _ 80 and 0.1% (w / w) lecithin were combined. Then in NanoMill _ 0.01 in a 10 ml chamber (NanoMill Systems, King of Prussia, PA), along with a 220 micron PolyMill _ Grinding media (Dow Chemical) (89% media loading) milled the mixture together. The mixture was milled at 5500 rpm for 60 minutes.
[0268] After milling, the particle size of the milled docetaxel particles was then measured in deionized distilled water using a Horiba LA 910 particle size analyzer. The mean particle size of the milled docetaxel particles was 166 nm, with a D50 of 147 nm and a D90 of 242 nm. Figure 3 shows a light micrograph of milled docetaxel.
[0269] This result indicates that stable nanoparticulate docetaxel formulations were successfully prepared with an average particle size of 166 nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com