Connecting peptide and polypeptide amalgamation representation method for polypeptide amalgamation representation
A technology for linking peptides and fusion peptides, applied in the fields of biotechnology and genetic engineering, can solve the problems of low specificity of chemical methods, limited use of peptides, and difficulty in popularization and application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
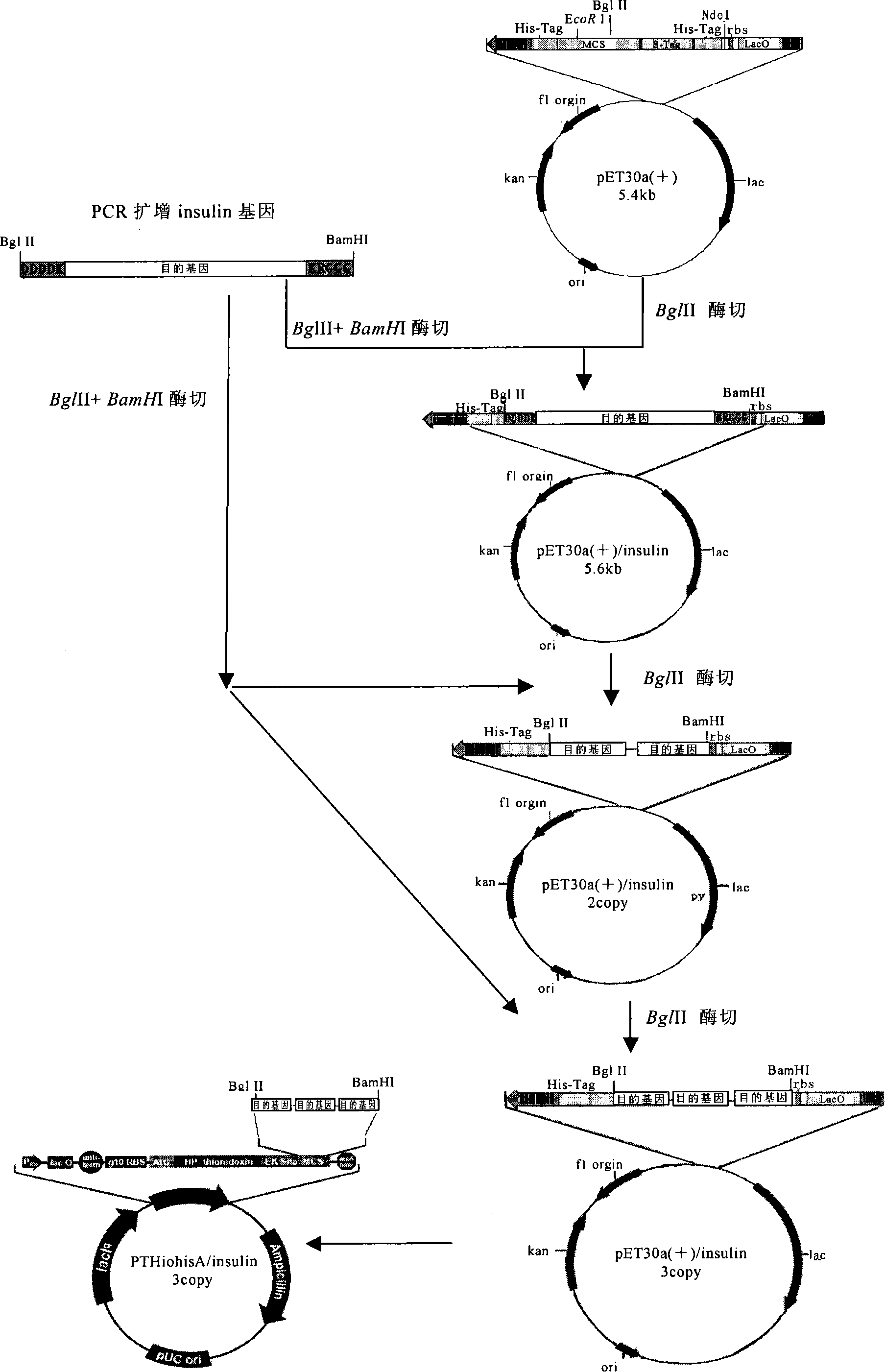
[0094] The preparation or expression method of the fusion polypeptide is well known to those skilled in the art, for example, the following steps can be used:
[0095] (1) The gene encoding the target polypeptide and the gene encoding the connecting peptide are connected in series according to the required copy number to form a fusion gene; the connection can be realized by designing appropriate primers, or can also be artificially synthesized The method for synthesizing the required fusion gene;
[0096] (2) inserting the fusion gene prepared in (1) into the multiple cloning site of the expression vector to obtain an expression vector inserted with the fusion gene;
[0097] (3) transferring the expression vector inserted into the fusion gene obtained in (2) into a host cell to obtain a transformed host cell;
[0098] (4) cultivating transformed host cells to express the fusion polypeptide;
[0099] (5) Separating and obtaining the fusion polypeptide.
[0100] In a preferre...
Embodiment 1
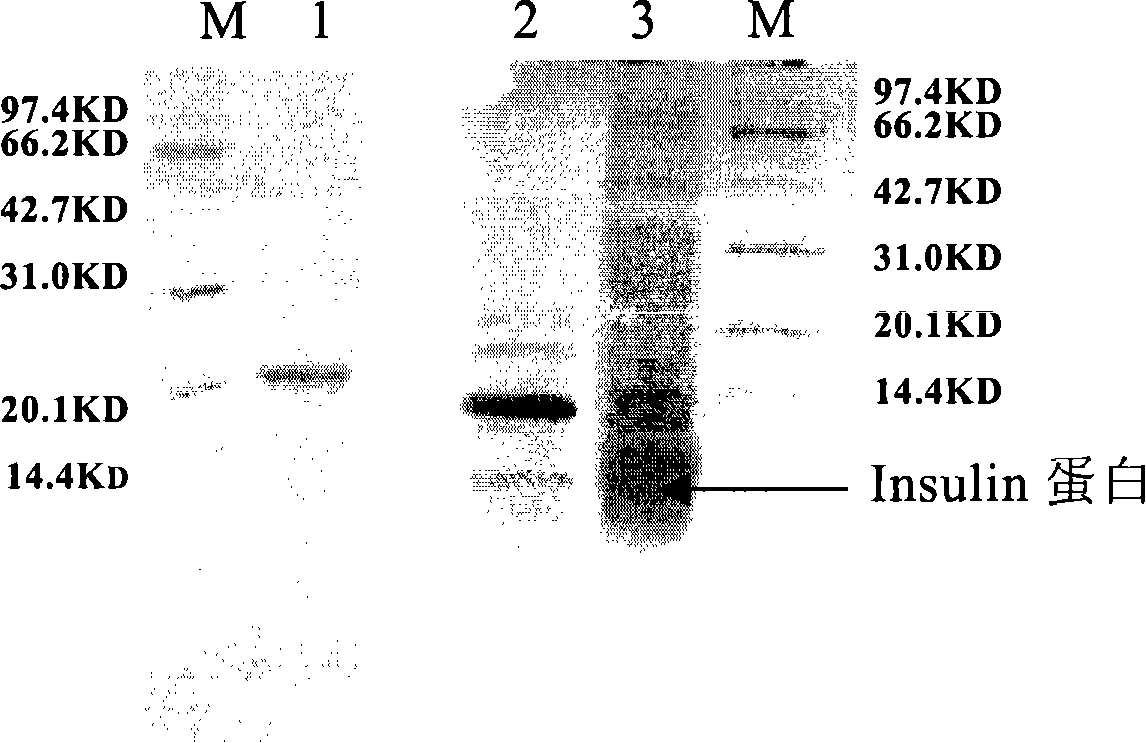
[0129] Example 1 Fusion expression of insulin (Insulin) and combined multi-enzyme digestion processing
[0130] In this example, the target polypeptides to be prepared are insulin A chain (Insulin A chain, GenBank accession number NM_000207) and insulin B chain (Insulin B chain, GenBank accession number NM_000207), and the linking peptide sequences used are as follows:
[0131]Arg-Ser-Asp-Asp-Asp-Asp-Lys (SEQ ID NO: 1), the sequence of its corresponding coding gene is AGA TCT GAT GAC GAT GAC AAA (SEQ ID NO: 2);
[0132] Lys-Arg-Ala-Asp-Asp-Asp-Asp-Lys (SEQ ID NO: 3), the sequence of its corresponding coding gene is AAG AGA GCT GAT GAC GAT GAT AAG (SEQ ID NO: 4);
[0133] Lys-Arg-Gly-Gly-Gly-Gly-Ser-Asp-Asp-Asp-Asp-Lys (SEQ ID NO: 5), the sequence of its corresponding coding gene is AAG AGA GGT GGA GGT GGA TCT GAT GAC GAT GAC AAA (SEQ ID NO: 6).
[0134] 1. Preparation of fusion polypeptide
[0135] The fusion polypeptide is designed as follows: Arg-Ser-Asp-Asp-Asp-Asp-...
Embodiment 2
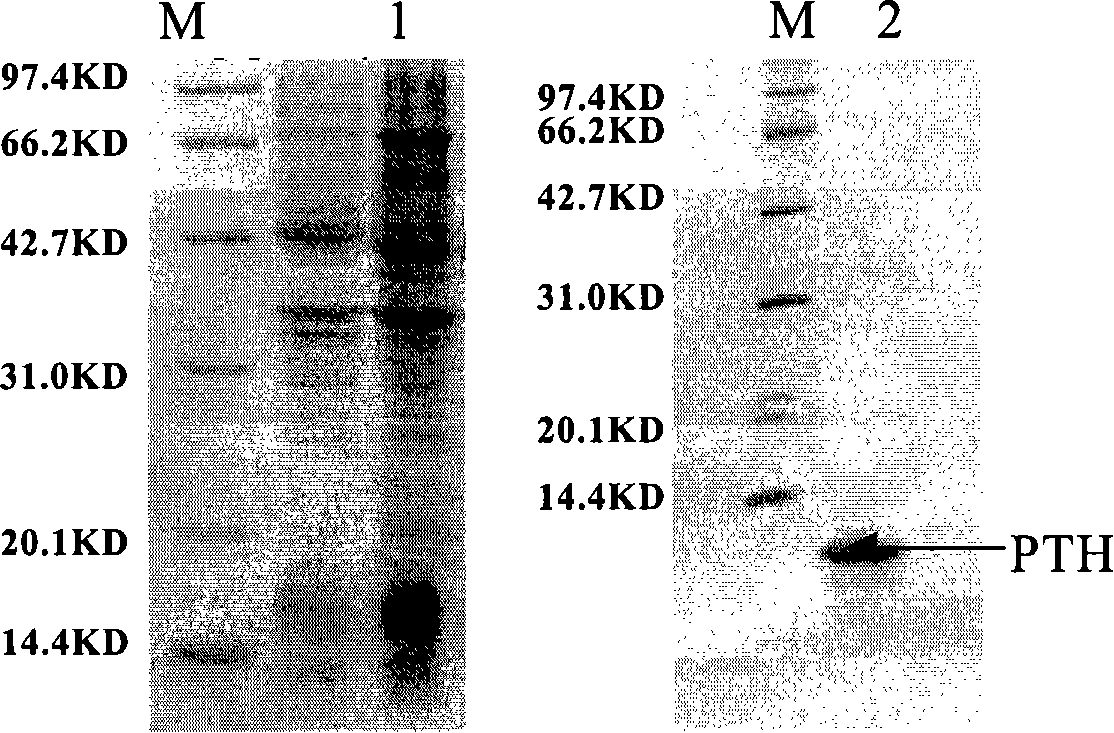
[0169] Example 2 Fusion expression of PTH and multi-enzyme combined enzyme digestion processing
[0170] In this example, the target polypeptide to be prepared is parathyroid hormone (PTH) (GenBank accession number NM_000315), and the linking peptide sequence used is as follows:
[0171] Arg-Ser-Asp-Asp-Asp-Asp-Lys (SEQ ID NO: 1);
[0172] Lys-Arg-Gly-Gly-Gly-Gly-Ser-Asp-Asp-Asp-Asp-Lys (SEQ ID NO: 5).
[0173] 1. Preparation of fusion polypeptide
[0174]Referring to a similar method as described in Example 1, the pTH gene was concatenated into a fusion gene containing 3 copies of the target gene and encoding the following fusion polypeptide by using the coding sequence of the aforementioned connecting peptide: Arg-Ser-Asp-Asp-Asp-Asp- Lys-PTH-Lys-Arg-Gly-Gly-Gly-Gly-Ser-Asp-Asp-Asp-Asp-Lys-PTH-Lys-Arg-Gly-Gly-Gly-Gly-Ser-Asp-Asp-Asp- Asp-Lys-PTH-Lys-Arg copy.
[0175] The sequencing analysis results are correct. Insert the fusion gene containing 3 copies of the pTH gene...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com