Hydrotalcite type hydrocracking catalyst and method for preparing same
A hydrogenation catalyst and hydrocracking technology, applied in chemical instruments and methods, hydrogen, inorganic chemistry, etc., can solve the problems of low crystallinity, large particle size, poor dispersibility, etc., and achieve high selectivity and high reaction. active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
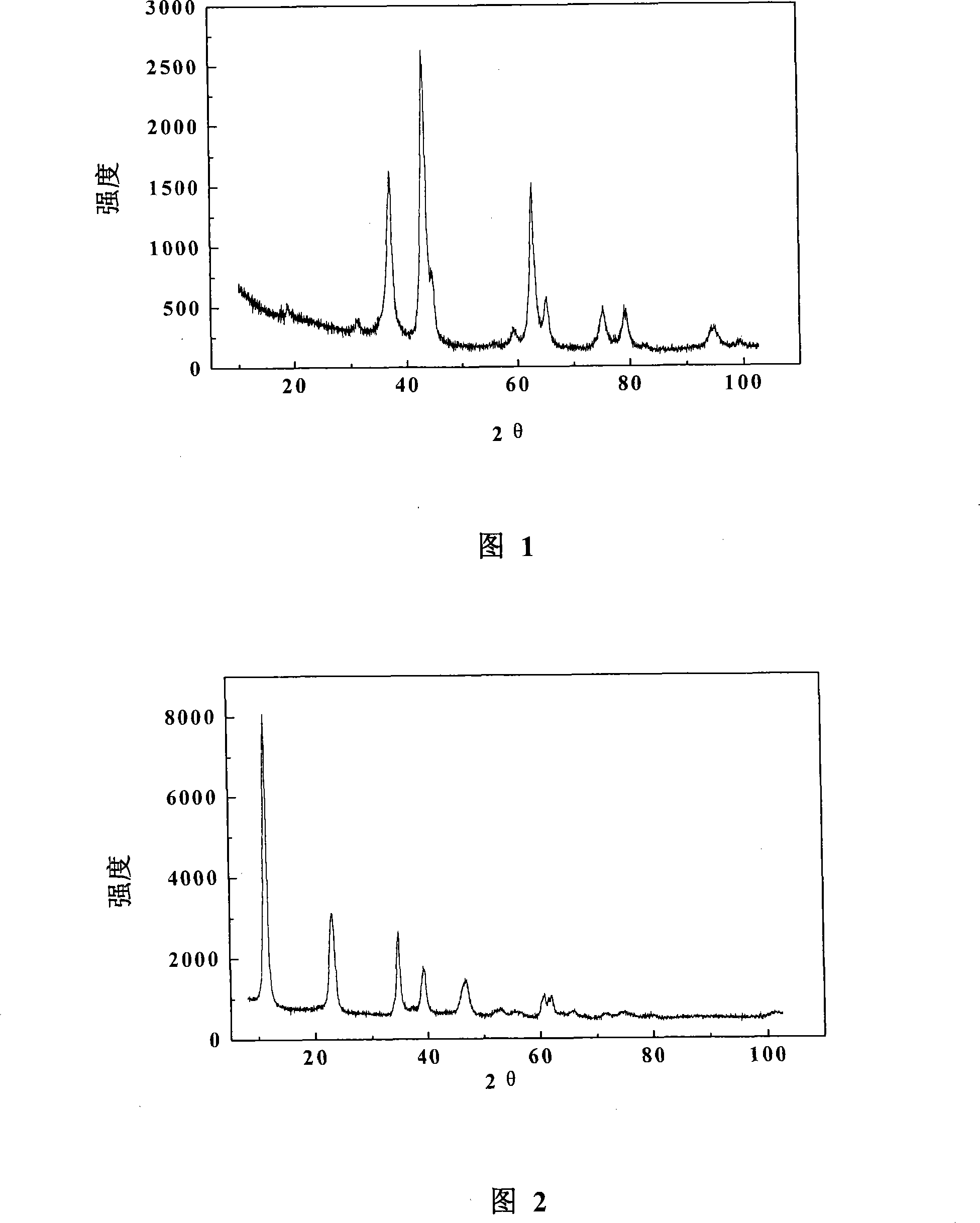
[0019] At room temperature, 50mmol Ni(NO 3 ) 2 ·6H 2 O, 250mmol Mg(NO 3 ) 2 ·6H 2 O, 100mmolAl(NO 3 ) 3 9H 2 O and 2.7 mol of urea were dissolved in a three-necked flask equipped with 250ml of deionized water, and the three-necked flask was immersed in a 105°C oil bath under vigorous stirring. After dynamic crystallization for 10 hours, in order to ensure that the urea was completely decomposed, it Under static aging for 18 hours. After the aging is complete, filter with suction, wash with deionized water for 3 times, dry the obtained sample at 95°C for 20 hours, then roast the dried sample at 500°C and 800°C for 16 and 3 hours respectively, crush and sieve, and take 20-40 range as a catalyst. The molecular formula of the prepared catalyst is Ni 0.5 Mg 2.5 AlO δ .
Embodiment 2
[0021] At room temperature, 50mmol Ni(NO 3 ) 2 ·6H 2 O, 50mmol Co(NO 3 ) 2 ·6H 2 O, 200mmolMg(NO 3 ) 3 ·6H 2 O, 100mmol Al(NO 3 ) 3 9H 2 O and 2.7mol of urea were dissolved in a three-necked flask equipped with 300ml of deionized water, and the three-necked flask was immersed in a 110°C oil bath under vigorous stirring. After dynamic crystallization for 12 hours, in order to ensure that the urea was completely decomposed, the temperature Under static aging for 18 hours. After the aging is complete, filter with suction, wash with deionized water for 3 times, dry the obtained sample at 95°C for 20 hours, then roast the dried sample at 550°C and 800°C for 15 and 4 hours respectively, crush and sieve, and take 20-40 range as a catalyst. The molecular formula of the prepared catalyst is Ni 0.5 co 0.5 Mg 2 AlO δ .
Embodiment 3
[0023] At room temperature, 50mmol Ni(NO 3 ) 2 ·6H 2 O, 50mmol Fe(NO 3 ) 3 9H 2 O, 200mmolMg(NO 3 ) 2 ·6H 2 O, 100mmol Al(NO 3 ) 3 9H 2 O and 3.5 mol of urea were dissolved in a three-necked flask equipped with 300ml of deionized water, and the three-necked flask was immersed in a 105°C oil bath under vigorous stirring. After dynamic crystallization for 15 hours, in order to ensure that the urea was completely decomposed, it Under static aging for 15 hours. After the aging is complete, filter with suction, wash with deionized water three times, dry the obtained sample at 95°C for 15 hours, then roast the dried sample at 600°C and 800°C for 18 hours and 3 hours respectively, crush and sieve, and take 20-40 range as a catalyst. The molecular formula of the prepared catalyst is Ni 0.5 Fe 0.5 Mg2 AlO δ .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com