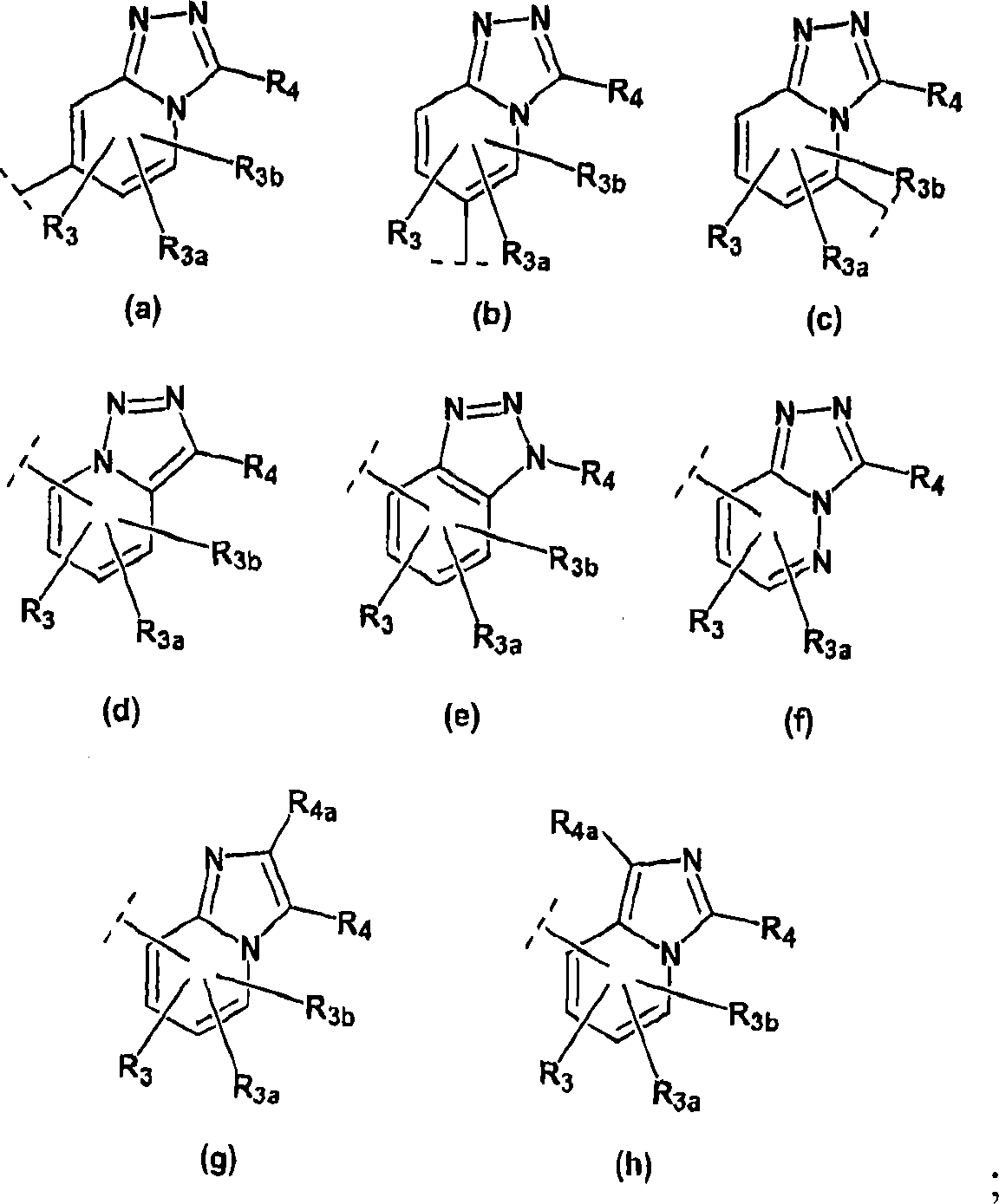
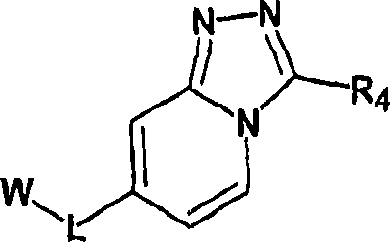
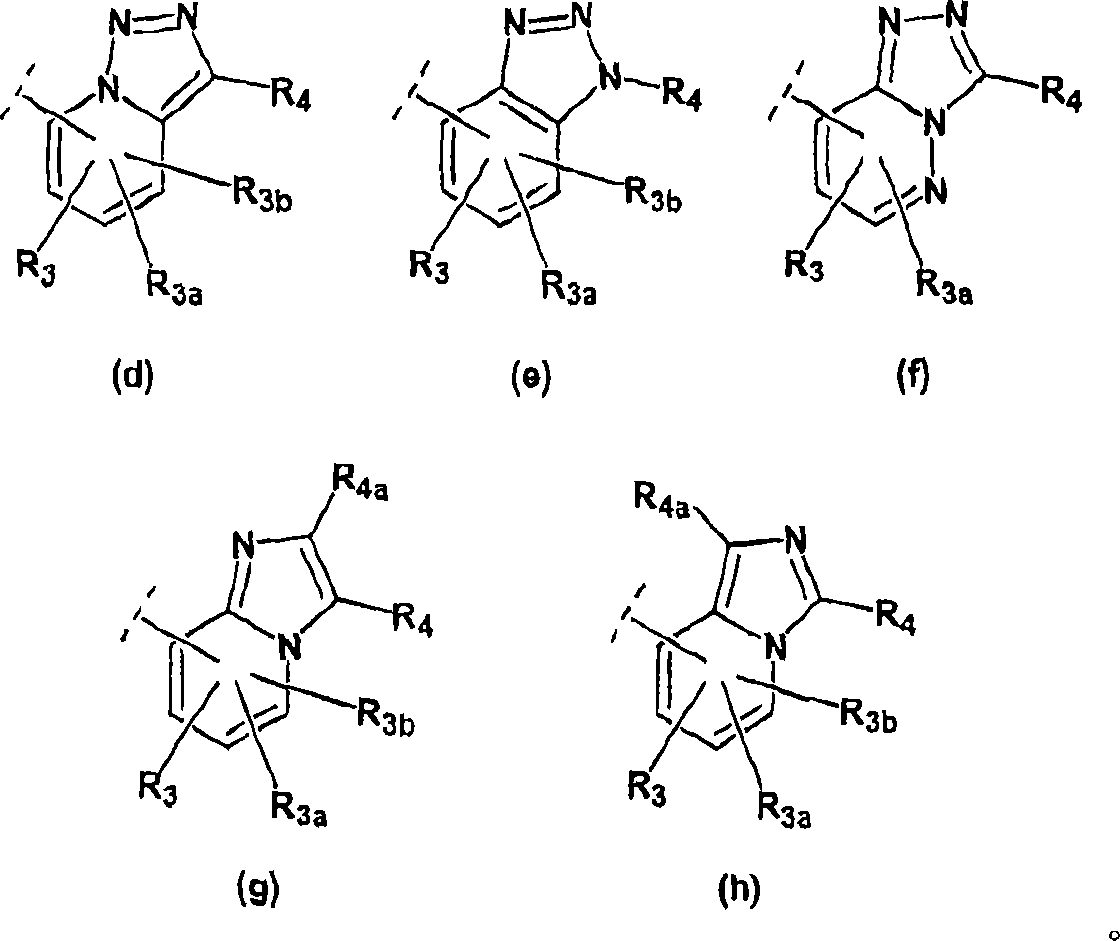
Imidazo- and triazolopyridines as inhibitors of 11-beta hydroxysteroid dehyftogenase type I
An aryl and alkyl technology, applied in the field of imidazopyridine and triazolopyridine as type I 11-β hydroxysteroid dehydrogenase inhibitors, can solve the problem of undisplayed plasma cortisol levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0362] 3-cycloheptyl-8-((2,6-dichlorophenoxy)methyl)H-imidazo[1,5-a]pyridine
[0363]
[0364] Compound 1A: 3-(Bromomethyl)picoline nitrile
[0365]
[0366] To a solution of 3-methylpicolininitrile (660 mg, 5.6 mmol) in 20 mL of carbon tetrachloride was added NBS (1 g, 5.6 mmol) and benzoyl peroxide (200 mg, 0.83 mmol) at room temperature. The reaction mixture was heated at 80 °C for 2 hours and then cooled to room temperature. The resulting solid was filtered off and the filtrate was concentrated under reduced pressure to yield a residue. The residue was diluted with ethyl acetate, washed with water, washed with Na 2 SO 4 It was dried and concentrated under reduced pressure to give crude product. The crude product was purified via silica gel chromatography (10% ethyl acetate in hexanes) to yield Compound 1A as a pale yellow oil. HPLCR t (Method A): 1.72 min. LC / MS(m / z)=197(M+H) + .
[0367] Compound 1B: 3-((2,6-dichlorophenoxy)methyl)picolininitrile
[0368] ...
Embodiment 2
[0379] 3-cycloheptyl-8-((2,6-dichlorophenoxy)methyl)H-imidazo[1,2-a]pyridine
[0380]
[0381] Compound 2A: 2-Bromo-1-cycloheptylethanone
[0382]
[0383] To a solution of cycloheptanecarboxylic acid (1.5 g, 10.5 mmol) in 5 mL of dichloromethane was added oxalyl chloride (10.5 ml, 21 mmol, 2 M in dichloromethane), followed by a few drops of DMF at room temperature. The reaction mixture was stirred at room temperature for 1 hour and then concentrated under reduced pressure to yield an oil. The oil was dissolved in 10 mL dry THF. The resulting solution was cooled to 0 °C and a solution of trimethylsilyldiazomethane (6.8 mL, 13.6 mmol, 2 M in diethyl ether) was added at 0 °C. When the addition was complete, the mixture was stirred overnight at 0 °C. At the end of this period, 48% aqueous HBr (2.4 mL, 80.9 mmol) was added at 0 °C and the reaction mixture was stirred at 0 °C for 2 h. The reaction mixture was then quenched with 20% aqueous sodium carbonate. The solvent was...
Embodiment 3
[0390] 3-cycloheptyl-7-((2,6-dichlorophenoxy)methyl)-[1,2,3]triazolo[1,5-a]pyridine
[0391]
[0392] Compound 3A: (6-bromopyridin-2-yl)(cycloheptyl)methanone
[0393]
[0394] Add 30 mL THF to a dry three-neck round bottom flask. The flask was cooled to -78 °C and n-butyllithium (16.9 mL, 42.2 mmol, 2.5 N in hexanes) was added to the flask in one portion. A solution of 2,6-dibromopyridine (10 g, 42.2 mmol) in 70 mL THF was added slowly via addition funnel at -78 °C to give a dark green solution. When the addition was complete, the mixture was stirred for an additional 15 min at -78 °C. To the dark green solution was added cycloheptylnitrile over 1 min at -78 °C. The reaction mixture was then warmed to room temperature. Once at the specified temperature, 6 N HCl solution (55 mL, 330 mmol) was added and the reaction mixture was heated to reflux for 5 min, then stirred at room temperature for 30 min. The resulting solution was made basic by the addition of 1 N NaOH at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com