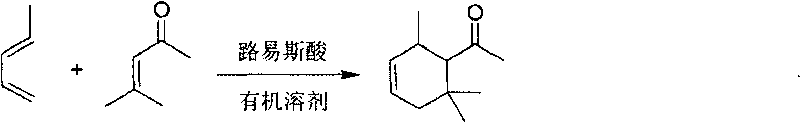
Method for preparing 1-(2,6,6-trimethylcyclohex 3-alkenyl) ethyl ketone
A technology of trimethylcyclohexyl and alkenyl, applied in the field of preparation of 1-ethanone, can solve the problems of unqualified aroma, poor product selectivity, low yield, etc., and achieve improved product yield, high recycling rate, Good solubility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 180g of nitroethane and 49g (0.5mol) of 4-methylpent-3-en-2-one into the reaction flask, stir well and then quickly add 50g (0.4mol) of anhydrous aluminum trichloride, and control the temperature for 5~ 20°C. After feeding, 125 g (1.8 mol) of 1,3-pentadiene was added dropwise, the reaction temperature was controlled at 25° C., the dropping time was controlled at 8 hours, and the reaction was continued for 3 hours after the dropwise addition was completed. The temperature dropped to 5°C, and washed with water until neutral. Recover the solvent and rectify under reduced pressure, collect the fraction at 78-82°C / 5mmHg to obtain 50 g of 1-(2,6,6-trimethylcyclohex-3-enyl)ethanone, with a yield of 60%.
Embodiment 2
[0021] Add 200g 1-nitropropane and 49g (0.5mol) 4-methylpent-3-en-2-one into the reaction flask, stir well and add 67g (0.5mol) anhydrous aluminum trichloride, control the temperature at 20°C . After feeding, 150 g (2.2 mol) of 1,3-pentadiene was added dropwise, the reaction temperature was controlled at 15° C., the dropping time was controlled at 10 h, and the reaction was continued for 3 h after the dropwise addition was completed. The temperature dropped to 5°C, and washed with water until neutral. Recover the solvent and rectify under reduced pressure, collect the fraction at 78-82°C / 5mmHg to obtain 58 g of colorless 1-(2,6,6-trimethylcyclohex-3-enyl)ethanone, with a yield of 70%.
Embodiment 3
[0023] Add 180g 2-nitropropane and 49g (0.5mol) 4-methylpent-3-en-2-one into the reaction flask, stir well and add 67g (0.5mol) anhydrous aluminum trichloride, control the temperature at 10°C . After feeding, 200 g (2.9 mol) of 1,3-pentadiene was slowly added dropwise, the reaction temperature was controlled at 18° C., the dropping time was controlled at 10 h, and the reaction was continued for 3 h after the dropwise addition was completed. The temperature dropped to 5°C, and washed with water until neutral. Recover the solvent and rectify under reduced pressure, collect the fraction at 78-82° C. / 5 mmHg to obtain 55 g of colorless 1-(2,6,6-trimethylcyclohex-3-enyl)ethanone with a yield of 66%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com