Novel heterocyclidene acetamide derivative
A technology of heterocyclic and hydrocarbon groups, applied in the field of new heterocyclic acetamide derivatives, can solve the problems of difficult oral administration, arrhythmia, low metabolic stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0041] [1] The first embodiment of the present invention
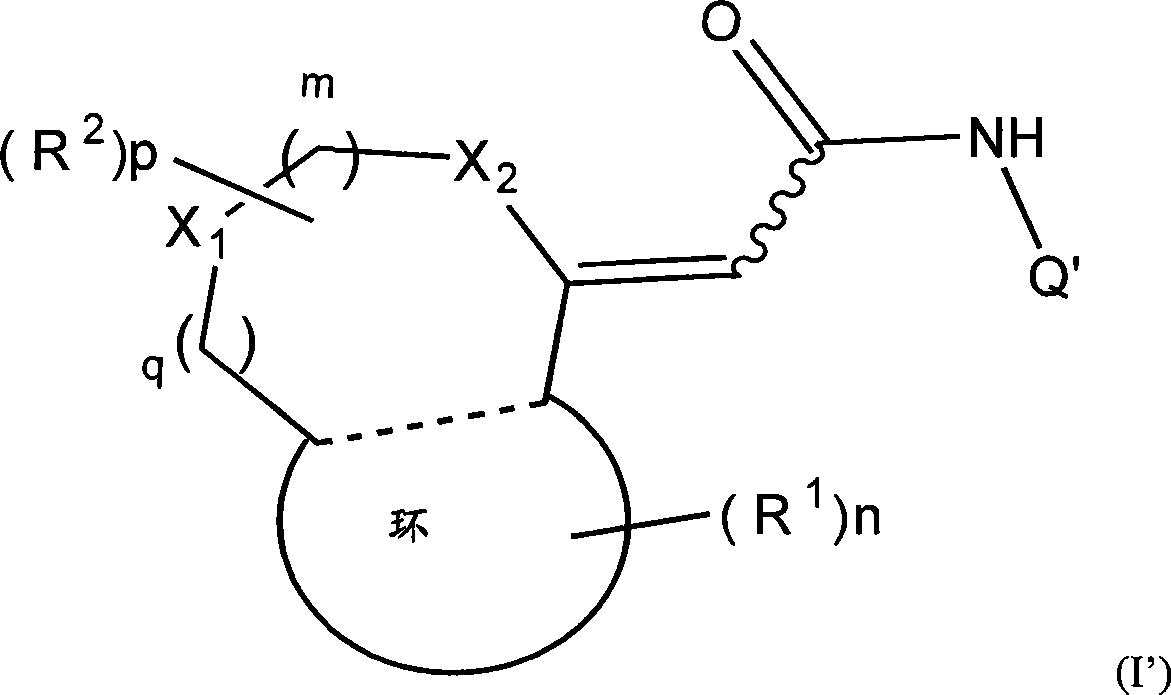
[0042] The first embodiment of the present invention provides a TRPV1 receptor antagonist comprising at least one compound represented by formula (I), its pharmaceutically acceptable salt and its solvate as an active ingredient:
[0043] [Ch.1]
[0044]
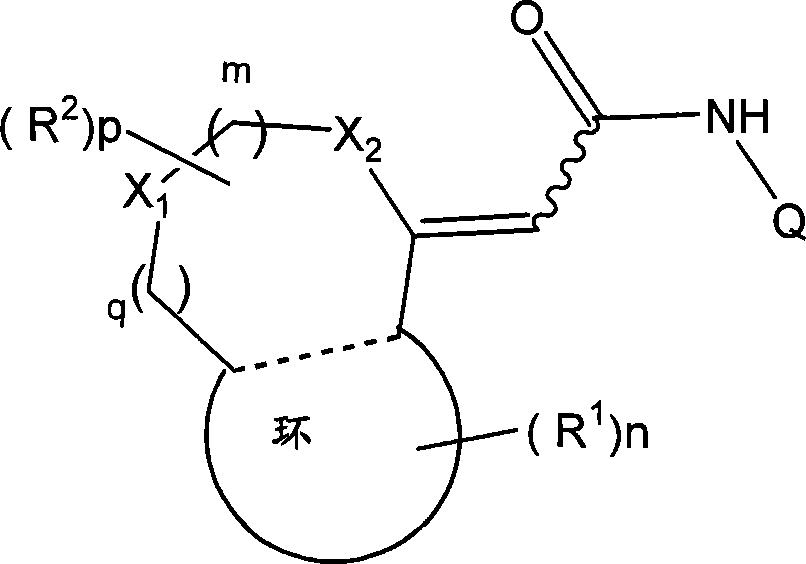
[0045] (wherein m, n and p each independently represent an integer of 0-2; q represents an integer of 0 or 1; R 1 Represents a group selected from the following groups: halogen atom, substituted or unsubstituted hydrocarbon group, substituted or unsubstituted heterocyclic group, substituted or unsubstituted C 1-6 Alkoxy, substituted or unsubstituted C 1-6 Alkoxycarbonyl, C which may be substituted or unsubstituted 1-6 Alkyl mono- or di-substituted amino, protected or unprotected hydroxyl, protected or unprotected carboxy, optionally substituted or unsubstituted C 1-6 Alkyl mono- or di-substituted carbamoyl, C 1-6 Alkanoyl, C 1-6 Alkylthio, C 1-6 Alkylsulfiny...
Embodiment approach 1-8-c
[0179] The groups of the specific examples of Q described in the embodiments [1-8-c] and [1-8-c] may each have no additional substituents, or may be further replaced by 1-3 selected from [1-1 - substituents in the categories (a-1)-(g-1) described in -a], or any substituents in specific examples can be exchanged. Among the groups listed in (a-1)-(g-1) above, "particularly preferred groups" include such substituents: such as C 1-6 Alkyl, C 2-6 Alkenyl, C 2-6 Alkynyl, halogen atom, halogenated C 1-6 Alkyl, cyano, amino, hydroxyl, carbamoyl, C 1-6 Alkoxy, C 2-6 Alkenyloxy, C 2-6 Alkynyloxy, C 1-6 Alkylthio, C 1-6 Alkylsulfinyl, C 1-6 Alkylsulfonyl, one / two C 1-6 Alkylamino, C 1-6 Alkoxycarbonyl, C 2-6 Alkanoyl, C 2-6 Alkanoylamino, Hydroxy-C 1-6 Alkyl, C 1-6 Alkoxy-C 1-6 Alkyl, carboxy-C 1-6 Alkyl, C 1-6 Alkoxycarbonyl-C 1-6 Alkyl, carbamoyl-C 1-6 Alkyl, N-C 1-6 Alkylcarbamoyl-C 1-6 Alkyl, N, N-diC 1-6 Alkylcarbamoyl-C 1-6 Alkyl, phenyl, phenoxy, phenylthio,...
Embodiment 102
[0337] (E)-2-(7-trifluoromethyl-2,3-dihydro-1-(cyclopentylacetyl)quinoline-4(1H)-ylidene)-N-(quinoline-7- base) acetamide (Example 102);
[0338] (E)-2-(7-trifluoromethyl-2,3-dihydro-1-cyclopentanecarbonylquinoline-4(1H)-ylidene)-N-(7-hydroxyl-5,6, 7,8-tetrahydronaphthalen-1-yl)acetamide (Example 105);
[0339] (E)-2-(7-trifluoromethyl-2,3-dihydroquinoline-4(1H)-ylidene)-N-(7-hydroxy-5,6,7,8-tetrahydronaphthalene -1-yl)acetamide (Example 106);
[0340](E)-2-(7-trifluoromethyl-2,3-dihydro-1-pentanoylquinoline-4(1H)-ylidene)-N-(3,4-dihydro-3-hydroxy (1H)quinolin-2-on-5-yl)acetamide (Example 107);
[0341] (E)-2-(7-trifluoromethyl-2,3-dihydro-1-pentanoylquinoline-4(1H)-ylidene)-N-(3-hydroxyl-1,2,3, 4-tetrahydroquinolin-5-yl)acetamide (Example 108);
[0342] (E)-2-(7-trifluoromethyl-2,3-dihydro-1-((2,2-dimethylcyclopropane)carbonyl)quinoline-4(1H)-ylidene)-N -(7-Hydroxy-5,6,7,8-tetrahydronaphthalen-1-yl)acetamide (Example 109);
[0343] (E)-2-(7-trifluoromethyl-2,3-dihydro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com