Cefathiamidine freeze-dried powder injection and preparing method thereof
A technology of cefathiamidine and freeze-dried powder injection, applied in the field of cefathiamidine freeze-dried powder injection and its preparation, can solve the problems of low product yield, frequent temperature changes, unstable moisture control, etc., and achieve consistent color and appearance The effect of plumping and improving the dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
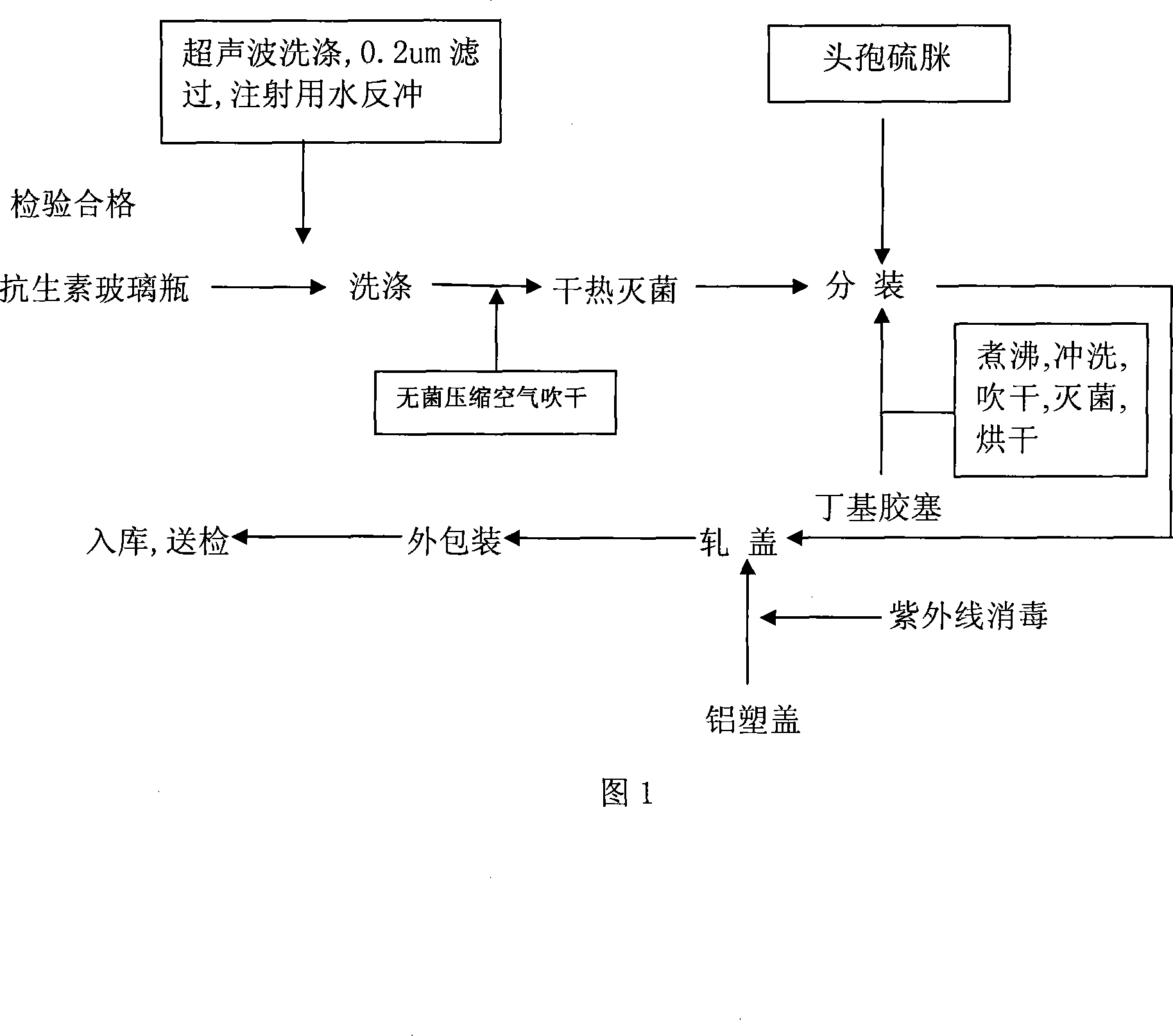
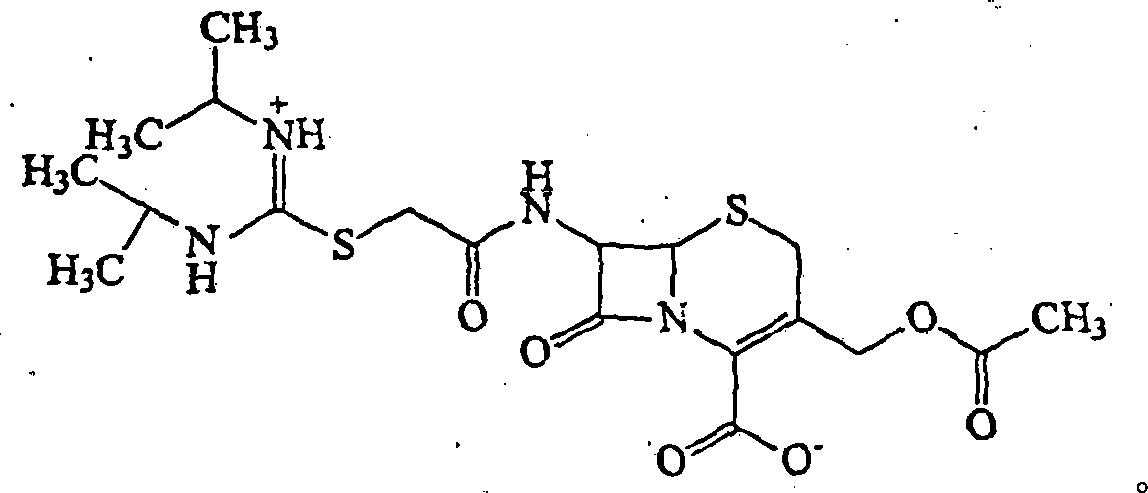
Image
Examples
Embodiment 1
[0038] Dissolve 40 g of cefathiamidine finished product in 50 mL of water for injection, heat to 50° C. and stir to dissolve, add 1 g of activated carbon and stir for 30 minutes to decolorize, then sterile filter and wash. Put the final filtrate into the tray of the freeze dryer, quickly cool down to -41°C, keep freezing at -41°C for 4 hours, then continue to cool down to -60°C, vacuumize, and gradually raise the temperature to -20°C within 6 hours ℃, maintain -20 ℃ and vacuum dry for 8 hours, continue to raise the temperature, rise to 25 ℃ within 2 hours, maintain 25 ℃ and vacuum dry until the vacuum degree does not change, to obtain 39.2g of cefathiamidine freeze-dried powder injection, the water content is 0.37% , yield 98%.
Embodiment 2
[0040] Dissolve 40 g of cefathiamidine finished product in 50 mL of water for injection, heat to 40°C and stir to dissolve, add 0.8 g of activated carbon and stir for 30 minutes to decolorize, then sterile filter and wash. Put the final filtrate into the feed tray of the freeze dryer, quickly cool down to -35°C, keep it frozen at -35°C for 5 hours, then continue to cool down to -50°C, vacuumize, and gradually raise the temperature to -18°C within 5 hours ℃, maintain -18 ℃ vacuum drying for 9 hours, continue to heat up, rise to 30 ℃ within 4 hours, maintain 30 ℃ vacuum drying until the vacuum degree does not change, obtain cefathiamidine freeze-dried powder injection 39.4, moisture is 0.40%, Yield 98.5%.
Embodiment 3
[0042] Dissolve 40 g of cefathiamidine finished product in 50 mL of water for injection, heat to 60°C and stir to dissolve, add 1.1 g of 769 type activated carbon and stir for 30 minutes to decolorize, then sterile filter and wash. Put the final filtrate into the tray of the freeze dryer, quickly cool down to -30°C, keep it frozen at -30°C for 3 hours, then continue to cool down to -55°C, vacuumize, and gradually raise the temperature to -15°C within 7 hours ℃, maintain -15 ℃ and vacuum dry for 7 hours, continue to heat up, rise to 28 ℃ within 2 hours, maintain 28 ℃ and vacuum dry until the vacuum degree does not change, to obtain cefathiamidine freeze-dried powder injection 39.1, the water content is 0.36%, Yield 98.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com