Process for producing pentaerythritol oleate
A technology of pentaerythritol oleate and esterification reaction, which is applied to the preparation of carboxylic acid esters, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of non-recycling, difficulty in catalyst recovery, etc., and achieve outstanding catalytic performance and easy Separation, less environmental pollution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
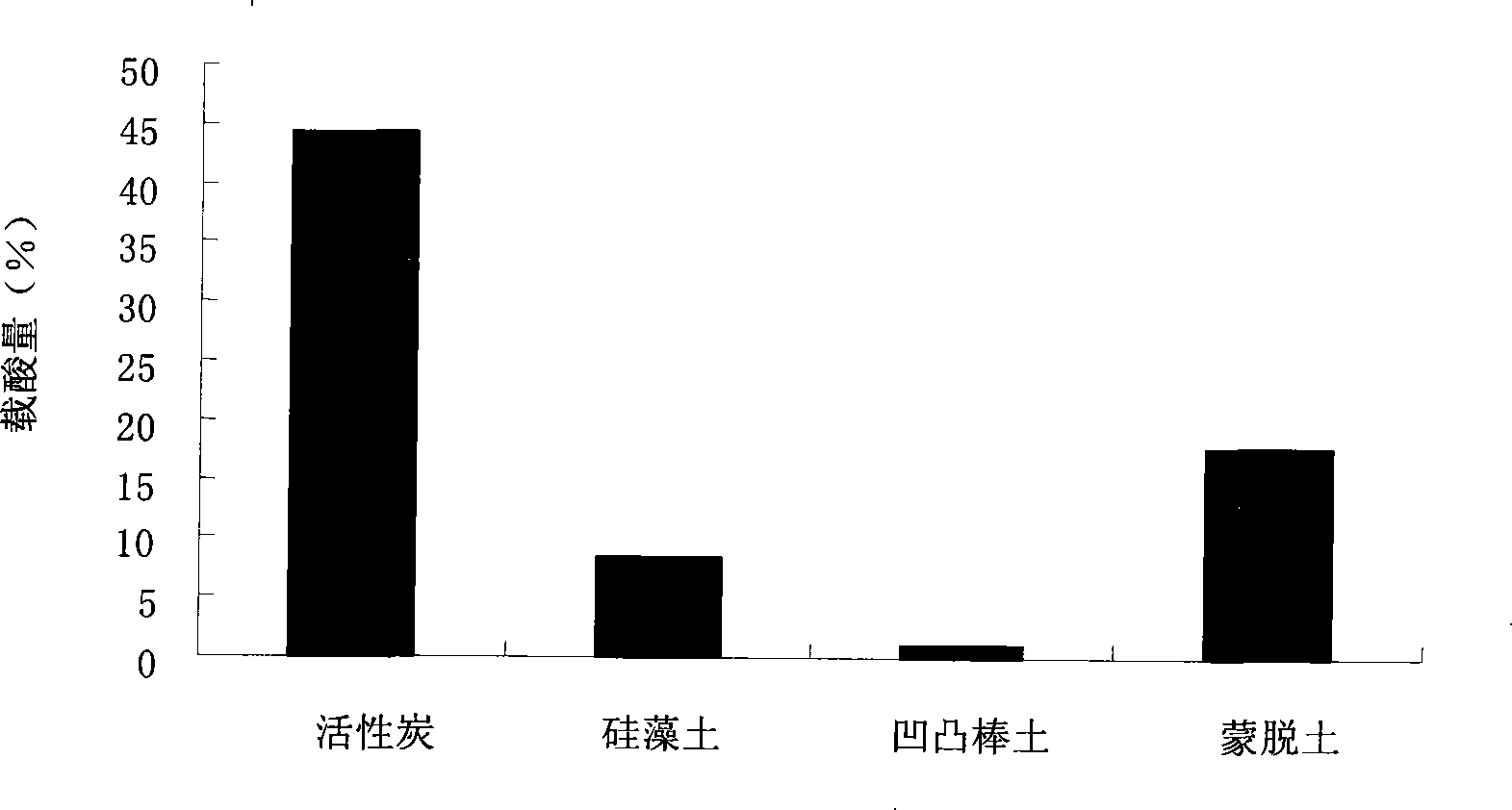
[0027] Example 1 The influence of different carriers on p-toluenesulfonic acid loading
[0028] The interaction between p-toluenesulfonic acid and the surface of the carrier directly affects the firmness of the load and the amount of acid supported. The esterification reaction uses the acidity of the catalyst. Therefore, the load of p-toluenesulfonic acid is related to the acidity and alkalinity of the carrier surface. The performance is closely related. If the surface of the carrier is too basic, the acidity of the catalyst will be greatly weakened or even lose its activity. The present invention compares the acid loading capacity of p-toluenesulfonic acid by activated carbon, diatomaceous earth, montmorillonite and attapulgite with a wide range of sources and low prices, and the results are as follows figure 1 Shown.
[0029] Structurally, activated carbon, diatomite, attapulgite and montmorillonite are all good carriers, but such figure 1 It is shown that under the same condi...
Embodiment 2
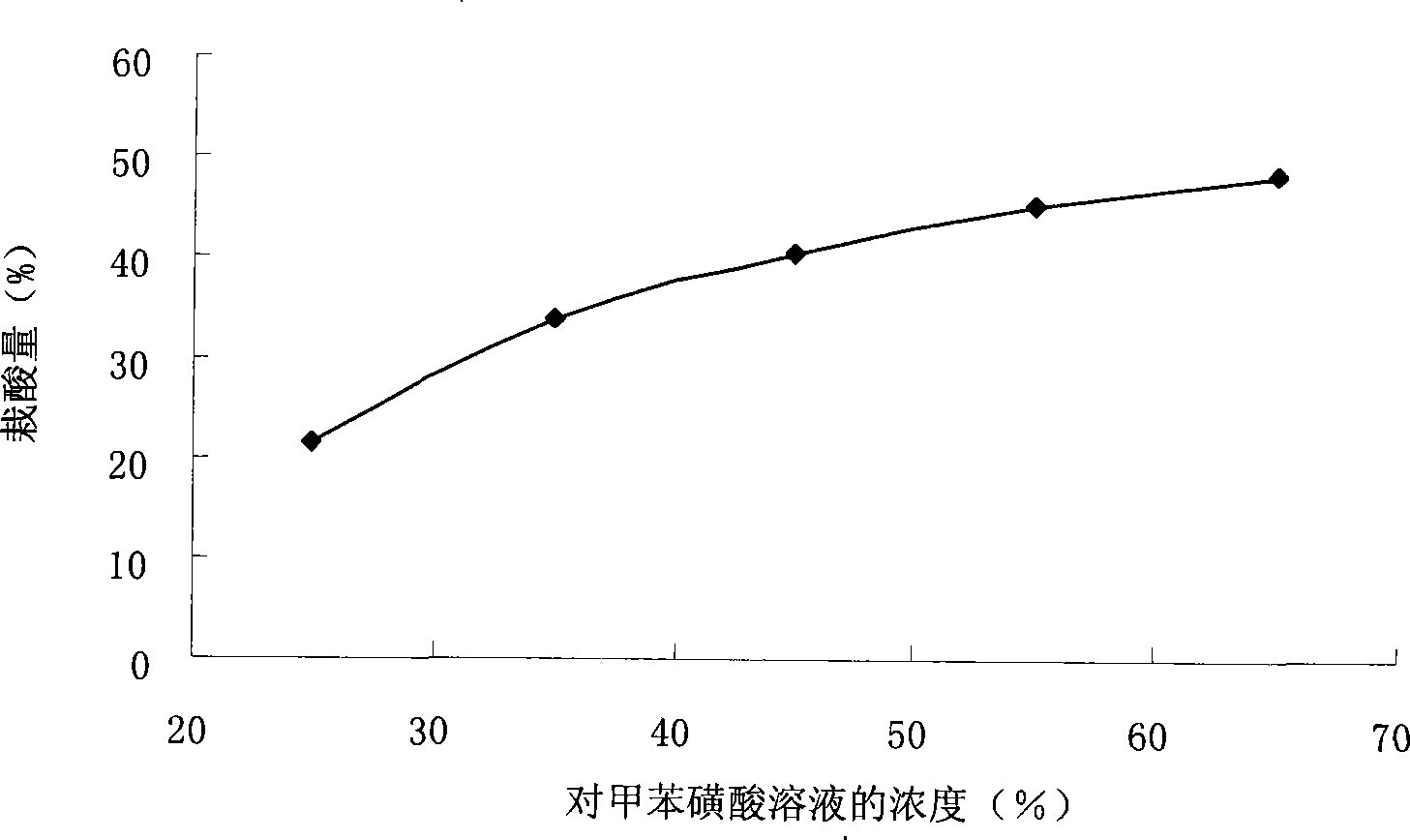
[0030] Example 2 The influence of the concentration of p-toluenesulfonic acid solution on the acid load
[0031] Weigh a certain amount of activated carbon and activate it at 120℃ for 2h. After cooling, weigh 10g and soak it in 60g solutions of p-toluenesulfonic acid with different concentrations (25%, 35%, 45%, 55%, 65%) for 42h , Filtration. Dry it at 110°C for 5 hours. After cooling, the catalysts with different acid loadings are obtained, which are put into the drying kettle for later use. Such as figure 2 As shown, the loading capacity increases with the concentration of the p-toluenesulfonic acid solution. When the concentration of the p-toluenesulfonic acid solution reaches 45%, the loading capacity increases slowly. The reasons may be as follows: The p-toluenesulfonic acid in the solution reaches a dynamic equilibrium, and the loading capacity no longer increases.
Embodiment 3
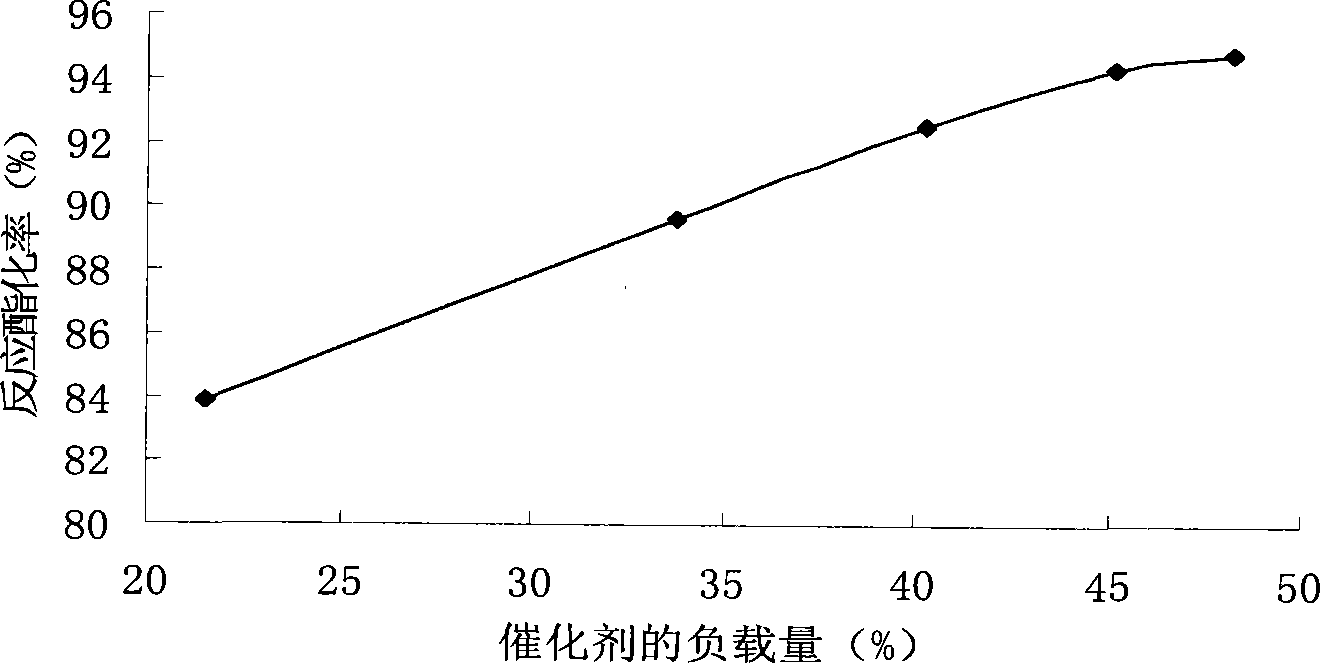
[0032] Example 3 Effect of different acid loading on the reaction
[0033] Take the same amount of catalysts with different loadings to react, the reaction conditions are oleic acid and pentaerythritol molar ratio 4:1, the amount of catalyst is 2% of the total mass of the reactants, and react at 140°C for 2 hours to obtain the acid-loaded catalyst and the ester of the product. A series of data corresponding to the conversion rate, such as image 3 As shown, the content of active components in the catalyst directly affects the activity of the catalyst, and the active components of the catalyst increase with the increase of the acid load. When the acid load reaches 45%, the increase in the esterification rate will be relatively gentle. The reason for this phenomenon may be that when the acid load is too large, the loss of active components is relatively large, and the esterification rate increases slowly. Therefore, about 45% of the acid loading is appropriate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pour point | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
| viscosity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com