Water-soluble triazine derivatives as well as preparation method and uses thereof
A s-triazine and water-soluble technology, which is applied in the field of water-soluble s-triazine derivatives and its preparation and application, to improve the separation effect, increase the separation ability, and achieve the effect of size separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
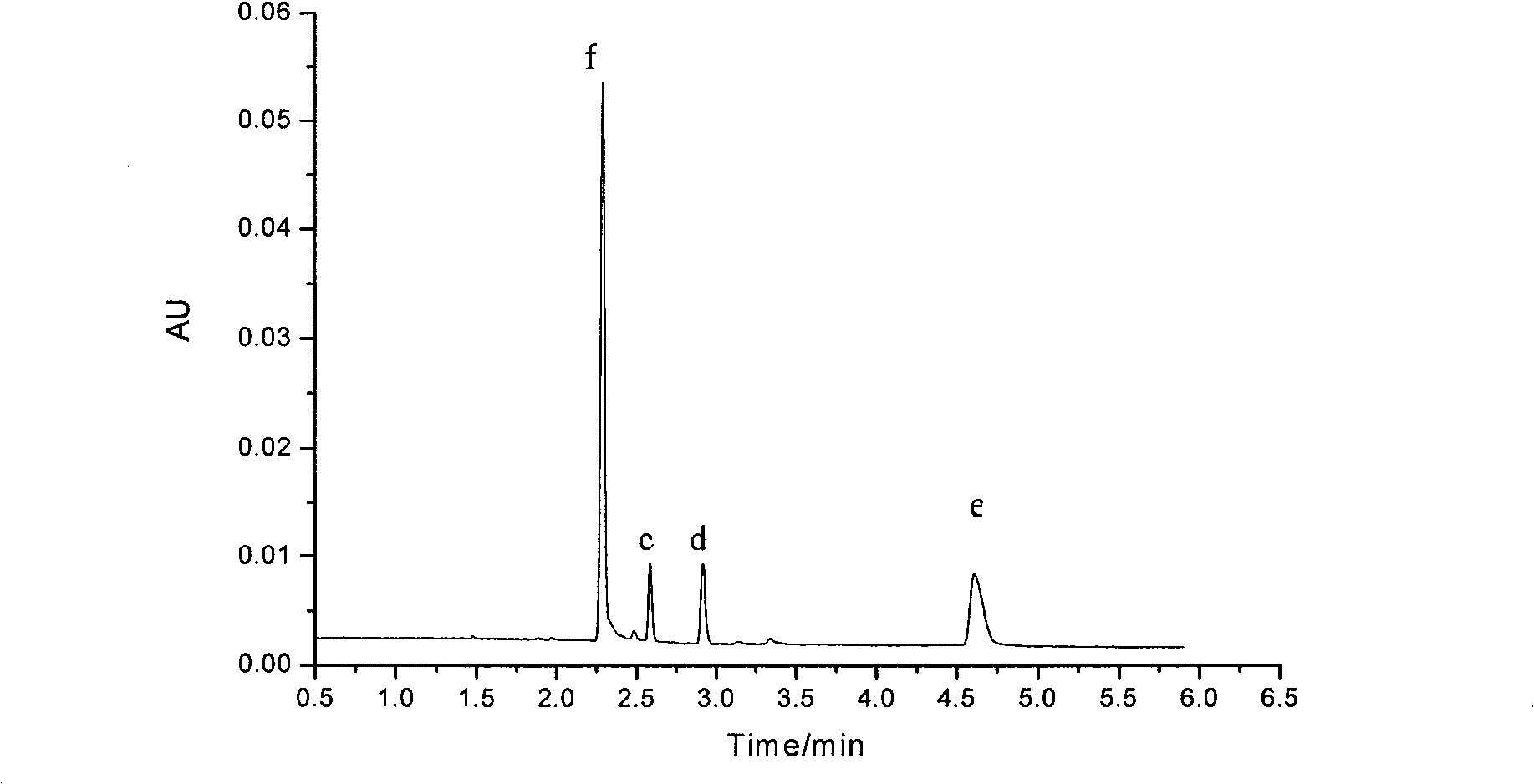
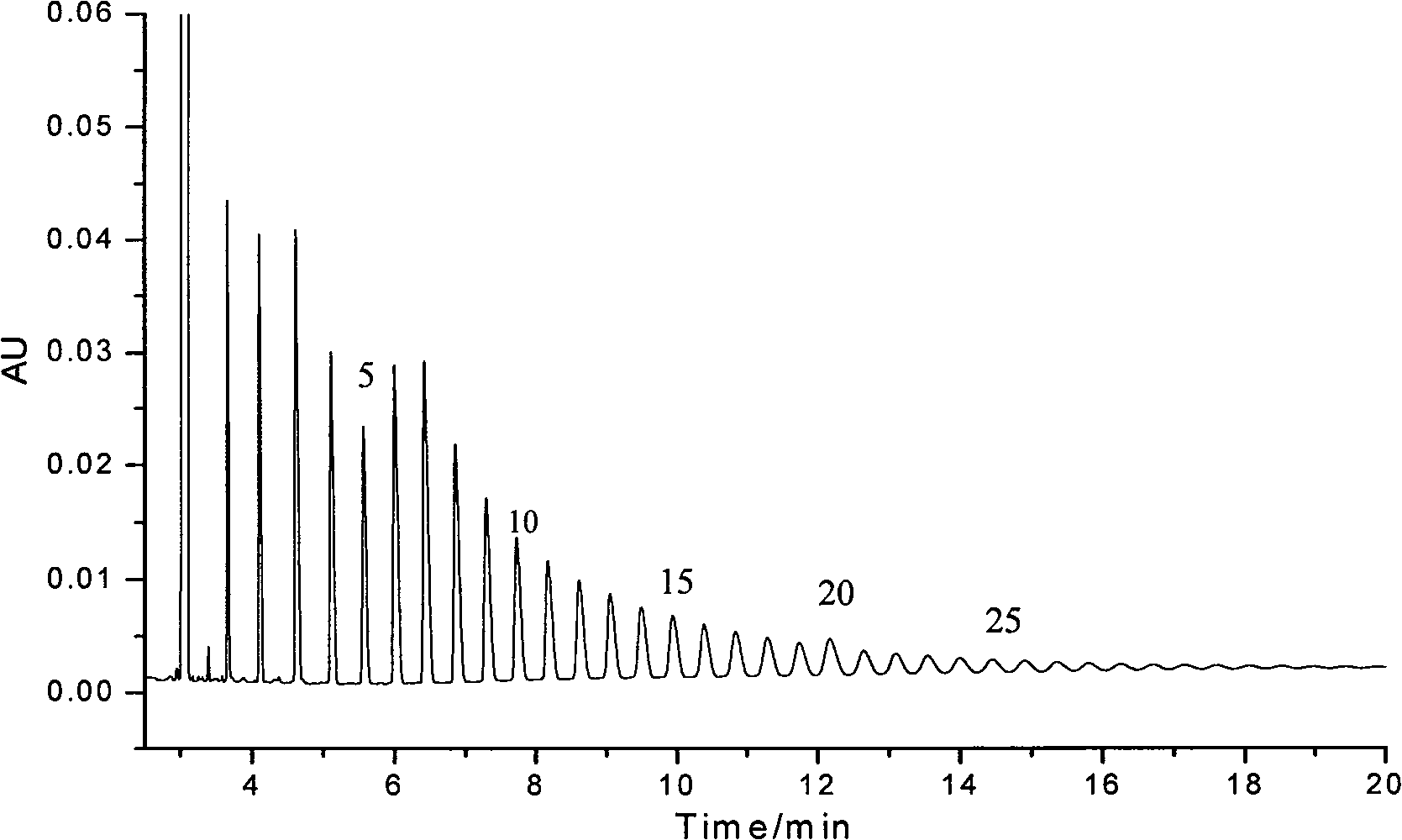
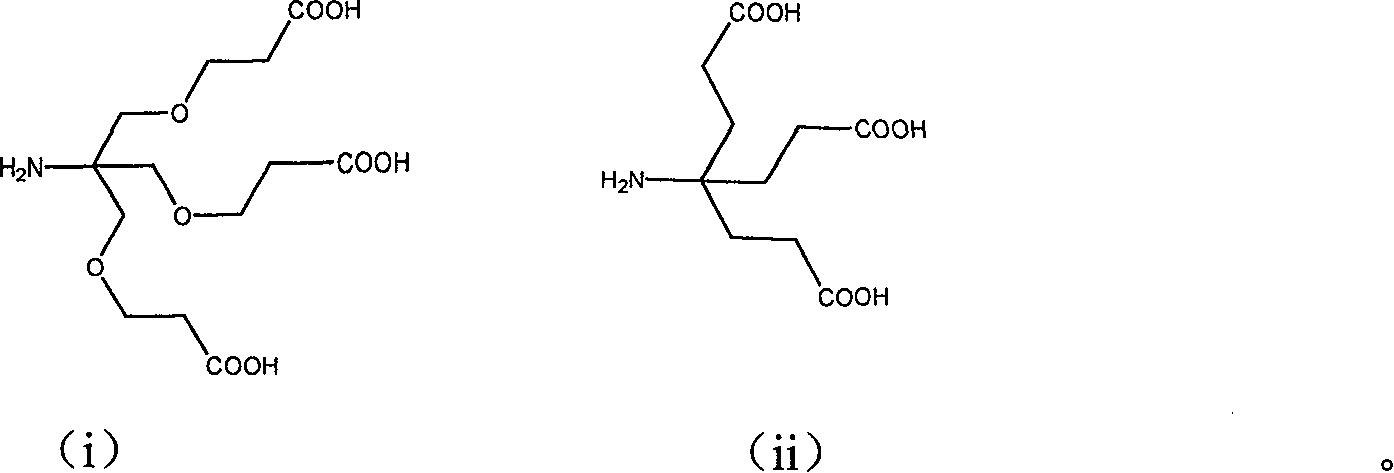
Image
Examples
Embodiment 1
[0027] Embodiment 1, the preparation of water-soluble halogenated s-triazine derivative (5)
[0028]
[0029] The reaction process is represented by the following reaction scheme:
[0030]
[0031] 2,5-Disulfonateaniline monosodium salt (16.5 g) was dissolved in 100 mL of NaOH (2.4 g) solution, and 40 g of crushed ice was added. Added hot acetone (10mL) solution of cyanuric chloride (5.5g) under the condition of vigorous stirring, reacted in ice-water bath for 1h, the temperature rose to 50°C, continued to react for 12h, added 20% NaOH solution during the reaction to make the reaction solution Keep it close to neutral. After the reaction, NaCl was added to precipitate the product 2-chloro-4,6-bis-(2,5-disulfonic acid aniline)-1,3,5-triazine (3), filtered and washed with 25% NaCl solution Cake 3 times, dry.
[0032] An aqueous solution (100 mL) of 2-chloro-4,6-bis-(2,5-disulfonateaniline)-1,3,5-triazine (3) (14.1 g) was added to phenylenediamine hydrochloride Salt (5....
Embodiment 2
[0033] Embodiment 2, the preparation of water-soluble chlorotriazine derivative (11)
[0034]
[0035] The reaction process is represented by the following reaction scheme:
[0036]
[0037] 2-Amino-5-hydroxy-1,7-naphthalenedisulfonic acid (16.0 g) was dissolved in 100 mL of NaOH (4 g) solution, and 100 g of crushed ice was added. In an ice-water bath, slowly add a solution of cyanuric chloride (4.58g) in hot acetone (10mL) under vigorous stirring. During the addition, use 20% NaOH solution to adjust the pH to 3, and react at this pH for 1h. The temperature was raised to 55° C., and the reaction was continued for 5 h. During the reaction, 20% NaOH solution was added to make the pH value of the reaction solution about 5. After the reaction, add NaCl to precipitate the product, filter, wash the filter cake with 25% NaCl solution, and dry.
[0038] Under stirring, 40 ml of a mixed solution of sodium nitrite (2.07 g) and sodium aniline-2,5-disulfonate (7 g) was added dropwis...
Embodiment 3
[0040] Embodiment 3, preparation of water-soluble chlorinated-s-triazine derivative (15)
[0041]
[0042] The reaction process is represented by the following reaction scheme
[0043]
[0044] The synthesis of 2-chloro-4,6-bis-(2,5-disulfonic acid aniline)-1,3,5-triazine (3) is as in Example 1.
[0045] An aqueous solution (60 mL) of 2-chloro-4,6-bis-(2,5-disulfonateaniline)-1,3,5-triazine (3) (14.1 g) was added to ethylenediamine hydrochloride In the salt (0.03mol) water (30mL) solution, 85 ° C water bath for 5h, during the reaction, add 20% NaOH solution to keep the reaction solution nearly neutral. The product (13) was precipitated by adding NaCl, filtered, washed and dried.
[0046] In an ice-water bath, add a hot acetone (5 mL) solution of cyanuric chloride (1.83 g) to an ice-cold (13) (7.5 g) aqueous solution under vigorous stirring, react in an ice bath, and adjust the pH with 20% NaOH The value is equal to 4, and the temperature rises to 55° C. after 2 hours....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com