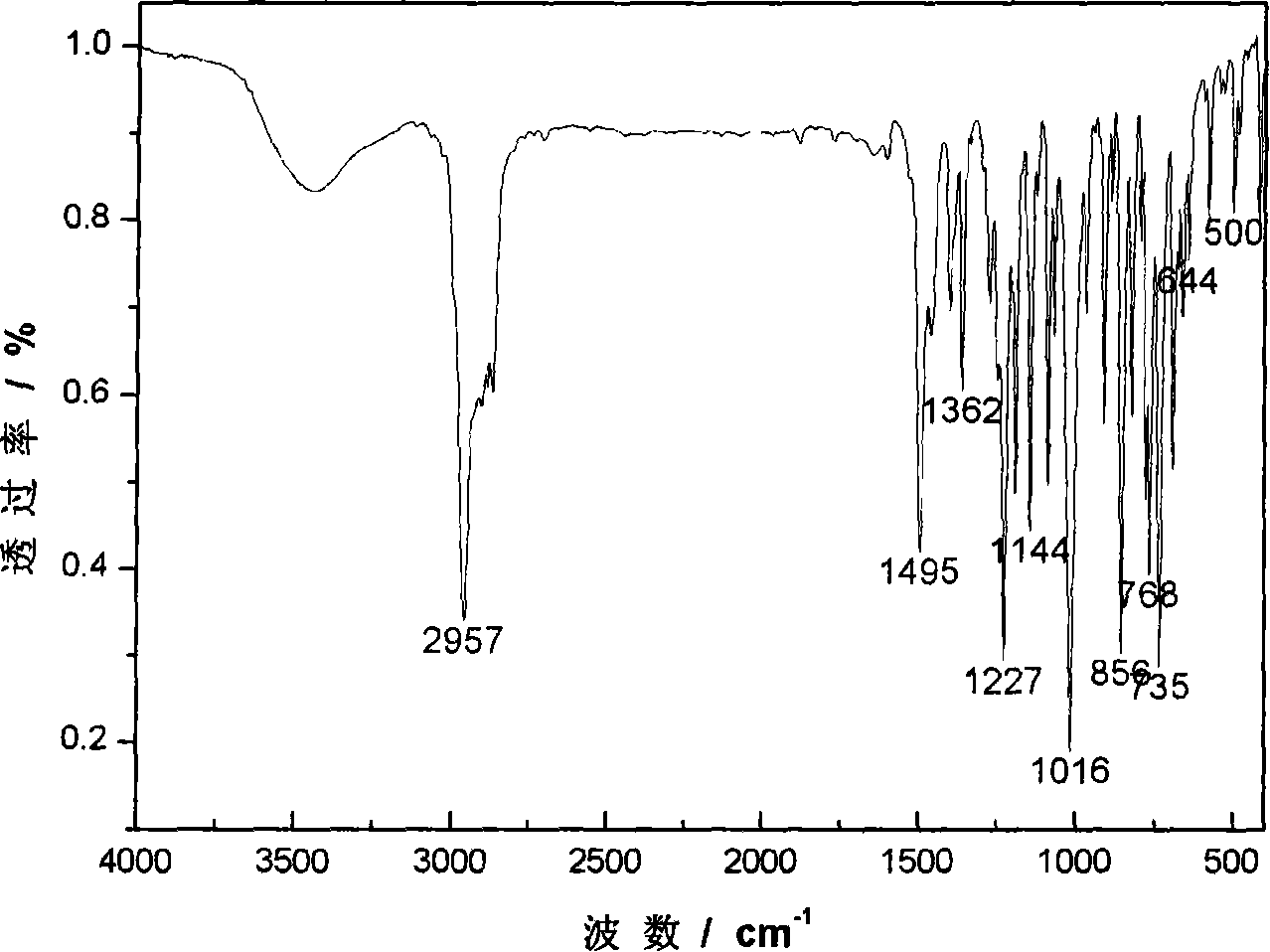
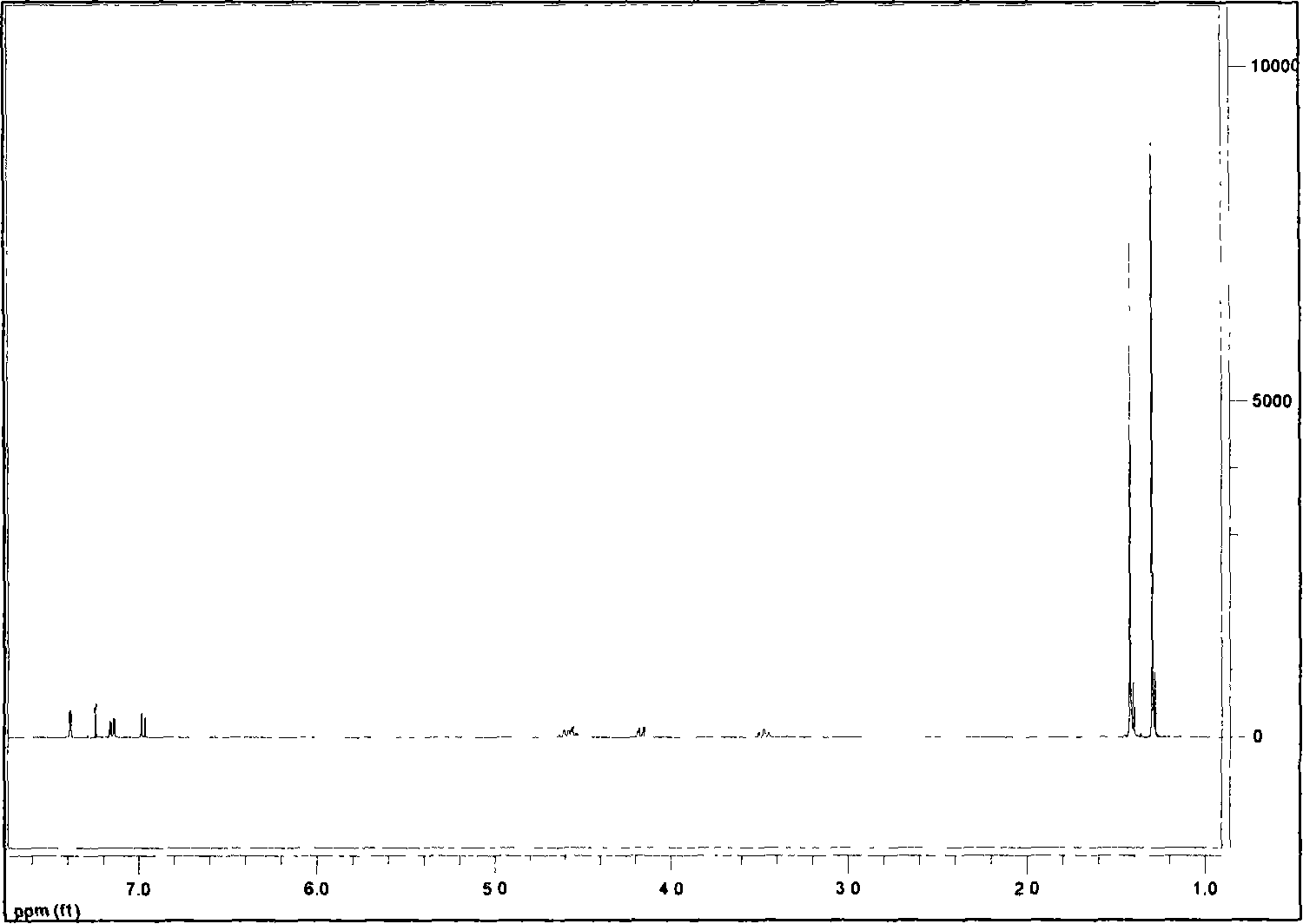
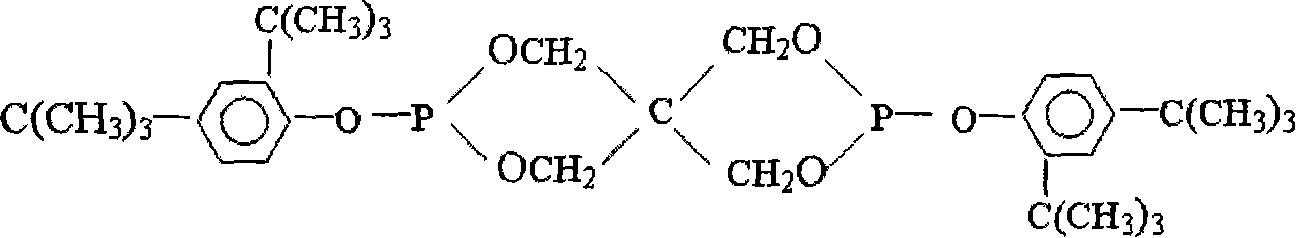
Method for synthesizing bis-(2,4-di-tert-butyl phenyl) pentaerythritol diphosphite
A technology of dichloropentaerythritol diphosphite and pentaerythritol diphosphite, which is applied in the field of synthesis of antioxidants, can solve the problems of harsh reaction conditions, difficult removal of phenol, and high cost of raw materials, and achieve easy reaction and low price. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The synthetic method of two (2,4-di-tert-butylphenyl) pentaerythritol diphosphite comprises the following steps:
[0025] (1) Synthesis of dichloropentaerythritol diphosphite;
[0026] In the there-necked flask equipped with stirrer, condenser, thermometer, receiver, add 250ml xylene solvent, 0.2mol pentaerythritol 27.2g and 0.27g catalyst, feed nitrogen, start stirring, stirring speed is 300 rpm, in Add 55g of 0.4mol phosphorus trichloride dropwise within 30 minutes, react at 30°C for 4h, and absorb the generated hydrogen chloride gas with 40g of sodium hydroxide solution; depressurize at a temperature of 90°C and a pressure of -0.001Mpa Unreacted phosphorus trichloride was distilled off, and 0.2 g of unreacted pentaerythritol was removed by filtration to obtain a xylene solution of dichloropentaerythritol diphosphite;
[0027] The mol ratio of phosphorus trichloride and pentaerythritol is 2.0: 1;
[0028] By weight, the consumption of the catalyst is 1% of pentaeryt...
Embodiment 2
[0032] The synthetic method of two (2,4-di-tert-butylphenyl) pentaerythritol diphosphite comprises the following steps:
[0033] 1. Synthesis of dichloropentaerythritol diphosphite;
[0034] In a three-necked flask equipped with a stirrer, a condenser, a thermometer, and a receiver, add 250ml of xylene solvent, 27.2g of 0.2mol pentaerythritol and 0.55g of catalyst methylammonium chloride, feed nitrogen, start stirring, and the stirring speed is 500 RPM, drop 60.5g of 0.44mol phosphorus trichloride within 40 minutes, react at 60°C for 3h, and absorb the hydrogen chloride gas generated with 40g of sodium hydroxide solution; at a temperature of 90°C and a pressure of -0.004 Under the condition of Mpa, unreacted phosphorus trichloride is distilled off under reduced pressure, filters out unreacted pentaerythritol 0.1g, obtains the xylene solution of dichloropentaerythritol diphosphite; The mol ratio of phosphorus trichloride and pentaerythritol is: 2.20: 1; By weight, the consumpt...
Embodiment 3
[0038] The synthetic method of two (2,4-di-tert-butylphenyl) pentaerythritol diphosphite comprises the following steps:
[0039] 1. Synthesis of dichloropentaerythritol diphosphite;
[0040] In a three-necked flask equipped with a stirrer, a condenser, a thermometer, and a receiver, add 230ml of xylene solvent, 27.2g of 0.2mol pentaerythritol and 0.9g of catalyst triethylamine, feed nitrogen, start stirring, and the stirring speed is 700 rpm In 50 minutes, 66g of 0.48mol phosphorus trichloride was added dropwise, reacted at 90°C for 1h, and the generated hydrogen chloride gas was absorbed with 40g of sodium hydroxide solution; under the condition of temperature of 90°C and pressure of -0.006Mpa Under reduced pressure, unreacted phosphorus trichloride was distilled off, and 0.2 g of unreacted pentaerythritol was removed by filtration to obtain a xylene solution of dichloropentaerythritol diphosphite; the molar ratio of phosphorus trichloride to pentaerythritol was 2.4:1 ; By w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com