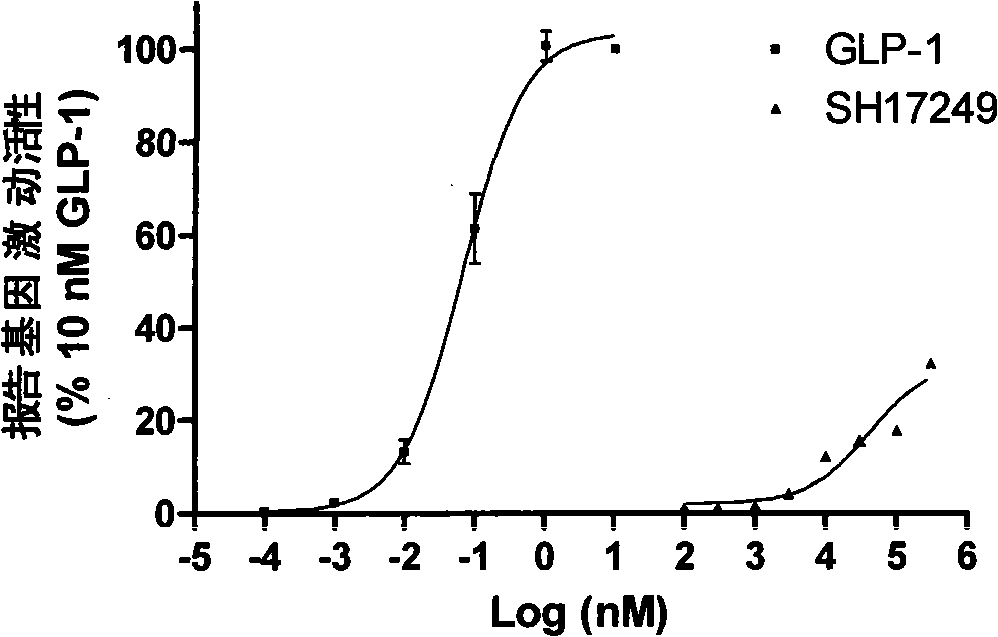
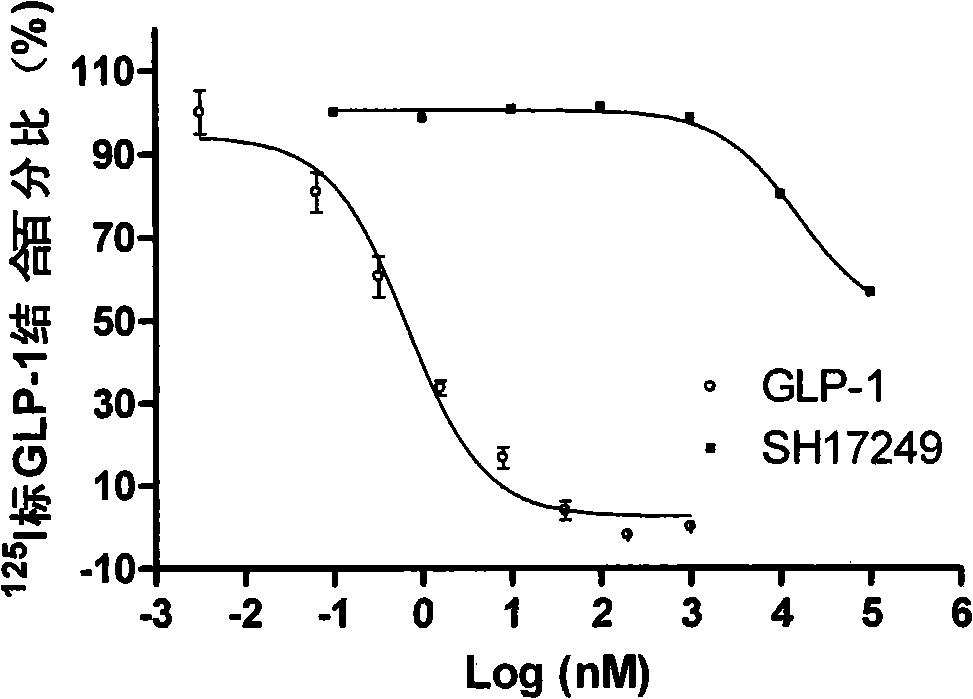
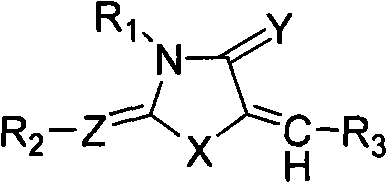
Substitutive five membered heterocyclic compound, preparation and medical use thereof
A kind of technology of five-membered heterocycle and compound, applied in the field of substituted five-membered heterocycle compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0193] The preparation of embodiment 1,2-(p-tolyl imino)-thiazol-4-ketone
[0194] NMR calibration: δH / C 7.26 / 77.0ppm (CDCl 3 ); δH / C 2.50 / 39.51ppm (DMSO-d 6 ).
[0195]
[0196] Add 17g (0.22mol) NH 4 Dissolve SCN in 100ml of acetone, stir at room temperature, slowly add 28.2g (0.2mol) of benzoyl chloride dropwise, a white solid appears, and the solution gradually turns yellow and thickens. After the dropwise addition, reflux for half an hour. Slowly add a solution of 21.43g (0.2mol) p-toluidine in acetone (50ml) dropwise, the rate of addition should make the solution slightly boil. After the dropwise addition, continue to reflux for half an hour and cool down.
[0197] The reaction solution was poured into 1.5L of water, stirred, and a yellow solid was precipitated. Suction filtration, the solid was dissolved in 30g NaOH aqueous solution (270ml), boiled for 5 minutes, cooled, and suction filtration to obtain 26.6g of solid (yield 80%).
[0198] Add 9.021g (0.054mol...
Embodiment 2
[0207] Embodiment 2, the preparation of N-methyl-2-(p-tolyl imino)-thiazol-4-one
[0208] NMR calibration: δH / C 7.26 / 77.0ppm (CDCl 3 ); δH / C 2.50 / 39.51ppm (DMSO-d 6 ).
[0209]
[0210] Add 1.4g (0.0191mol) CH 3 NCS, 2g (0.0187mol) of p-toluidine, 8ml of absolute ethanol, heated to reflux for 4 hours, cooled, crystallized, and suction filtered to obtain 2.92g of solid (yield 87%).
[0211] Add 2.92g (0.0162mol) N to a 100ml three-neck flask 1 -(p-tolyl)-N 2 - Methylthiourea, 4.60 g (0.0486 mol) of chloroacetic acid, 4 g (0.0486 mol) of sodium acetate, a small amount of TEBA, 100 ml of absolute ethanol. Heat to reflux overnight and filter while hot. The filter cake was washed twice with a small amount of absolute ethanol. The combined filtrates were concentrated to a small volume under reduced pressure. Cooling, crystallization, suction filtration, and drying to obtain 1.088 g of white-yellow solid (yield 30.5%).
[0212] 1 HNMR (300MHz, CDCl 3 ) δ 2.35 (s, 3H), 3...
Embodiment 3
[0229] Preparation of Example 3, 2-(p-tolylimino)-5-(2,4-dimethoxyphenylmethylene)-thiazol-4-one
[0230]
[0231]Add 1g (4.85mmol) 2-(p-tolylimino)-thiazol-4-one, 2.4g (14.55mmol) 2,4-dimethoxybenzaldehyde, 1.2g (14.55mmol) in 100ml one-port bottle Sodium acetate, 40ml glacial acetic acid, heated to reflux. The color of the reaction solution gradually deepened, and after reflux for 4 hours, cooling and crystallization occurred. After suction filtration, the filter cake was washed once with ethyl acetate and dried to obtain 0.8 g of orange solid (yield 46.6%).
[0232] 1 HNMR (300MHz, DMSO-d 6 )δ2.30(s, 3H), 3.34(s, NH), 3.80(s, 3H), 3.87(s, 3H), 6.65-6.73(m, 2H), 6.93-6.96(d, J=8.1Hz , 1H), 7.19-7.24 (t, J = 8.1 Hz, 3H), 7.39 (d, 1H), 7.65 (d, J = 8.1 Hz, 1H).
[0233] The following compounds were also synthesized by the above method:
[0234] 2-(p-tolylimino)-5-(p-N,N-dimethylaminophenylmethylene)-thiazol-4-one
[0235]
[0236] 1 HNMR (300MHz, DMSO-d 6 ) δ 2....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com