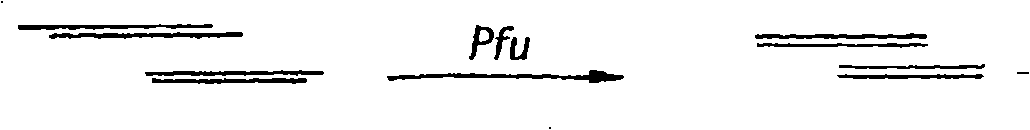Methods of amplifying and sequencing nucleic acids
一种核酸序列、测序的技术,应用在化学仪器和方法、生物化学设备和方法、通过光学手段进行材料分析等方向,能够解决昂贵、DNA测序仪慢、测序仪慢等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
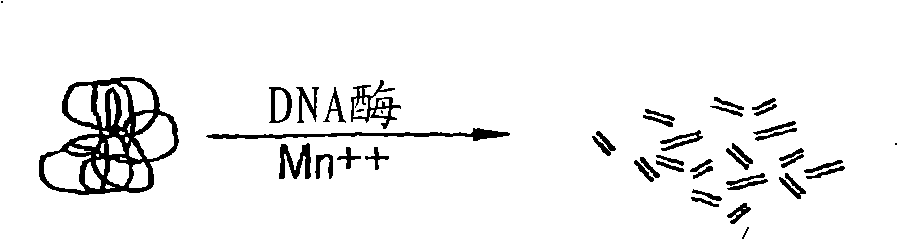
[0135] The attachment of the anchor primer to the solid or removable solid substrate can occur before, during, or after extension of the annealed anchor primer. Therefore, in one embodiment, one or more anchor primers are attached to a solid or removable fixed substrate, after which the anchor primers anneal to the target nucleic acid and are extended in the presence of a polymerase. Alternatively, in the second embodiment, the anchor primer is first annealed to the target nucleic acid, and the 3'OH end of the annealed anchor primer is extended with a polymerase. The extended anchor primer is then attached to a solid or removable solid substrate. By changing the sequence of the anchor primer, the unique target nucleic acid present in the nucleic acid population can be specifically amplified.
[0136]The following summarizes preferred embodiments for preparing template nucleic acids for amplification and sequencing reactions. The present invention includes a method for preparing sa...
Embodiment 1
[0389] Example 1: Sample preparation
[0390] DNA sample:
[0391] DNA should be of high quality and free of contaminants such as proteins, nucleic acids, lipids, and other chemicals (such as residual EDTA from preparations) and salts. The preferred genomic DNA should have a 260 / 280 ratio of 1.8 or higher. If it is necessary to sequence the genome of only one organism, the DNA should be quality checked to ensure that it does not contain contaminating DNA. For example, a preparation of human DNA can be tested by PCR to ensure that it is not contaminated by bacterial DNA molecules. Another way to test for contamination is to use a suitable probe known to be specific to a known organism (e.g. human or mouse) and a second probe known to be specific to a possible contaminating organism (e.g. Escherichia coli). Enzyme digestion mode, especially Southern blot after restriction enzyme digestion. If necessary, the DNA should be from a single clone of the organism (for example, if it is fro...
Embodiment 2
[0499] Example 2: Primer design
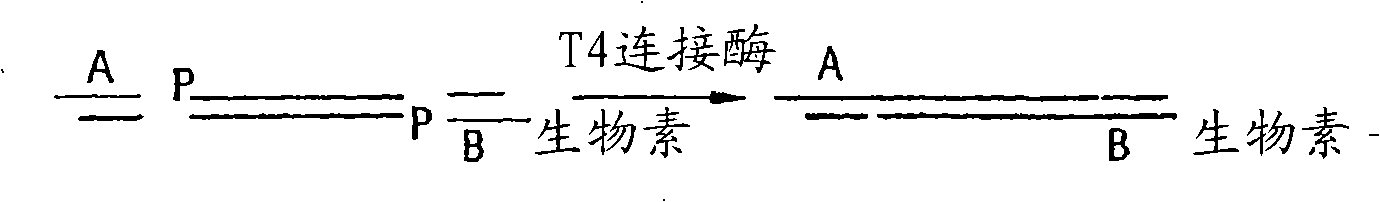
[0500] As discussed above, the universal adaptor is designed to include: 1) a set of unique PCR primer regions, whose length is typically 20 bp (adjacent to (2)); 2) a set of unique sequencing primer regions, whose length is typically 20 bp; And 3) optionally followed by a unique distinguishing key sequence, which consists of at least one of the four deoxyribonucleotides (ie A, C, G, T). The possible cross-hybridization between the primer and the unexpected region of the genome of interest increases as the size of the genome increases and the length of the complete match with the primer decreases. However, for the reasons explained below, this potential interaction with the cross-hybridization region (CHR) is not expected to cause problems.
[0501] In a preferred embodiment of the present invention, the single-stranded DNA library is used for PCR amplification and subsequent sequencing. The sequencing method requires random digestion of a given g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com