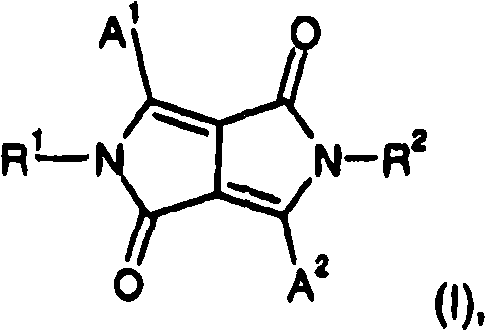
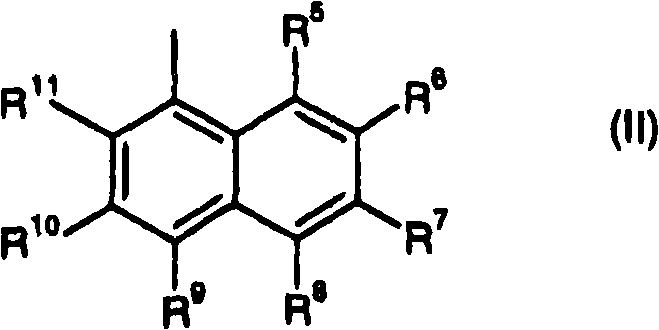
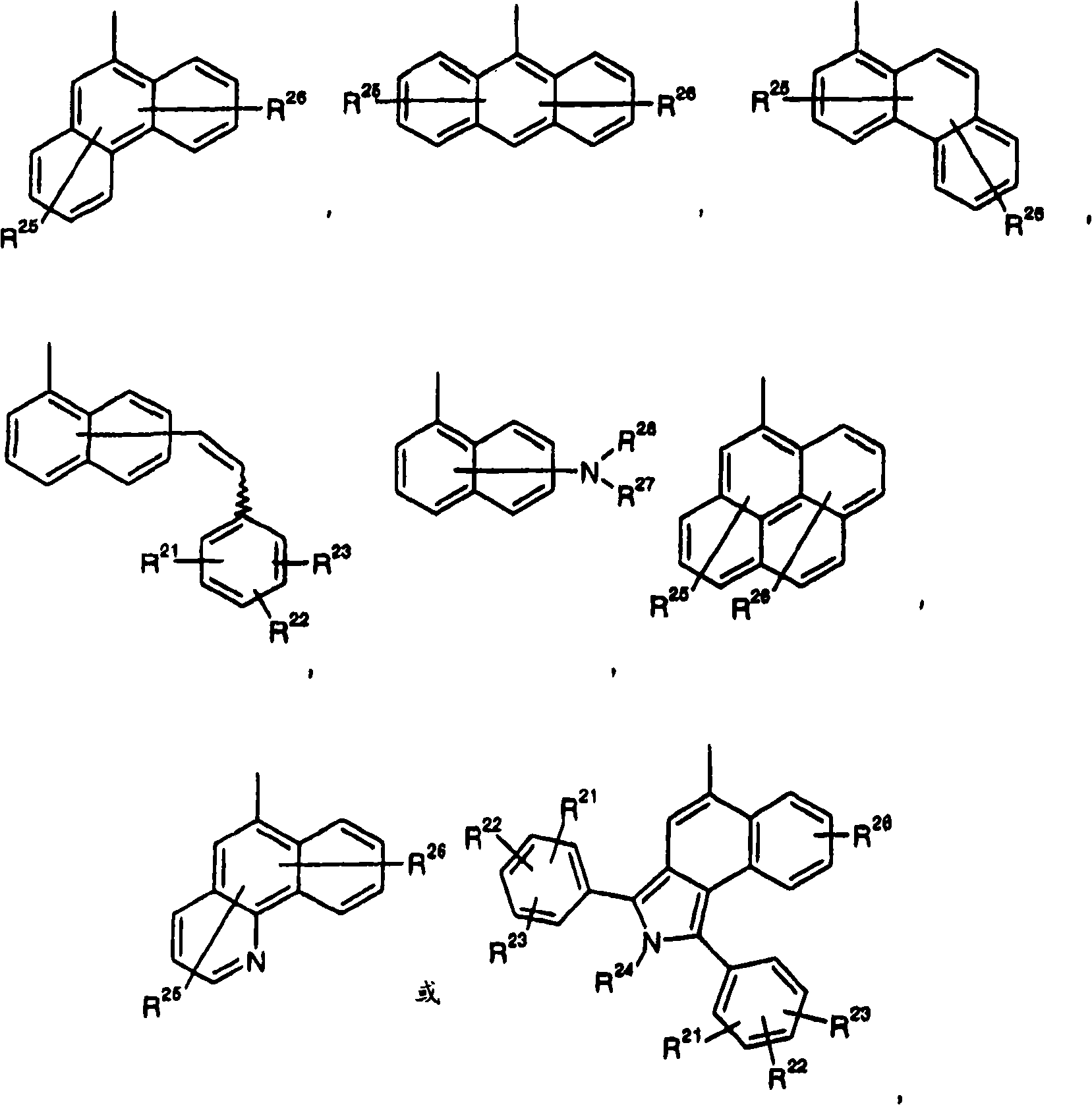
Fluorescent diketopyrrolopyrroles
A technology of diketopyrrolopyrrole and radicals, applied in diketopyrrolopyrrole dyes, optics, optical filters, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0203] 24.6 g (0.22 mol) of potassium tert-butoxide, 41 g (0.20 mol) of 9-cyanophenanthrene and 200 ml of t-amyl alcohol were heated to a maximum of 100° C. under a nitrogen atmosphere. Once this temperature was reached, a solution of 23 g (0.10 mol) of di-n-butylsuccinate and 70 ml of t-amyl alcohol was added within 1 hour using a dropping funnel. When the addition was complete, the reaction mixture was maintained at 100°C for 16 hours, cooled to 65°C, neutralized with 20 ml of glacial acetic acid and boiled briefly at reflux temperature. The resulting pigment suspension was filtered at room temperature. The filter cake was suspended in 300 ml of methanol and the pigment was isolated by filtration, finally washed with methanol and water until the washings became colorless, and then dried under vacuum at 100° C. to yield 8.5 g of 1,4-diketone-3 , 6-bis-(9-phenanthrenyl)-pyrrolo-(3,4-c)-pyrrole.
[0204] 2.2g (4.5mmol) 1,4-diketone-3,6-bis-(9-phenanthrenyl)-pyrrolo-(3,4-c)-py...
Embodiment 2
[0206] Example 1 was repeated except that 3,5-di-t-butylbenzyl bromide was used as an alkylating agent, thus obtaining a red solid (yield: 36%).
Embodiment 3
[0208] Example 1 was repeated except that methyl iodide was used as an alkylating agent, thus obtaining an orange solid (yield: 48%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com