Activated factor X(FXa) stimulants as new antihemorrhagic agents for topical use
A blood coagulation factor, local treatment technology, applied in anti-inflammatory agents, medical preparations containing active ingredients, blood diseases, etc., can solve the problems of lack of acute effect, risk of side effects, unstable fibrin clot, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0461] The results of Example 1 clearly show that:
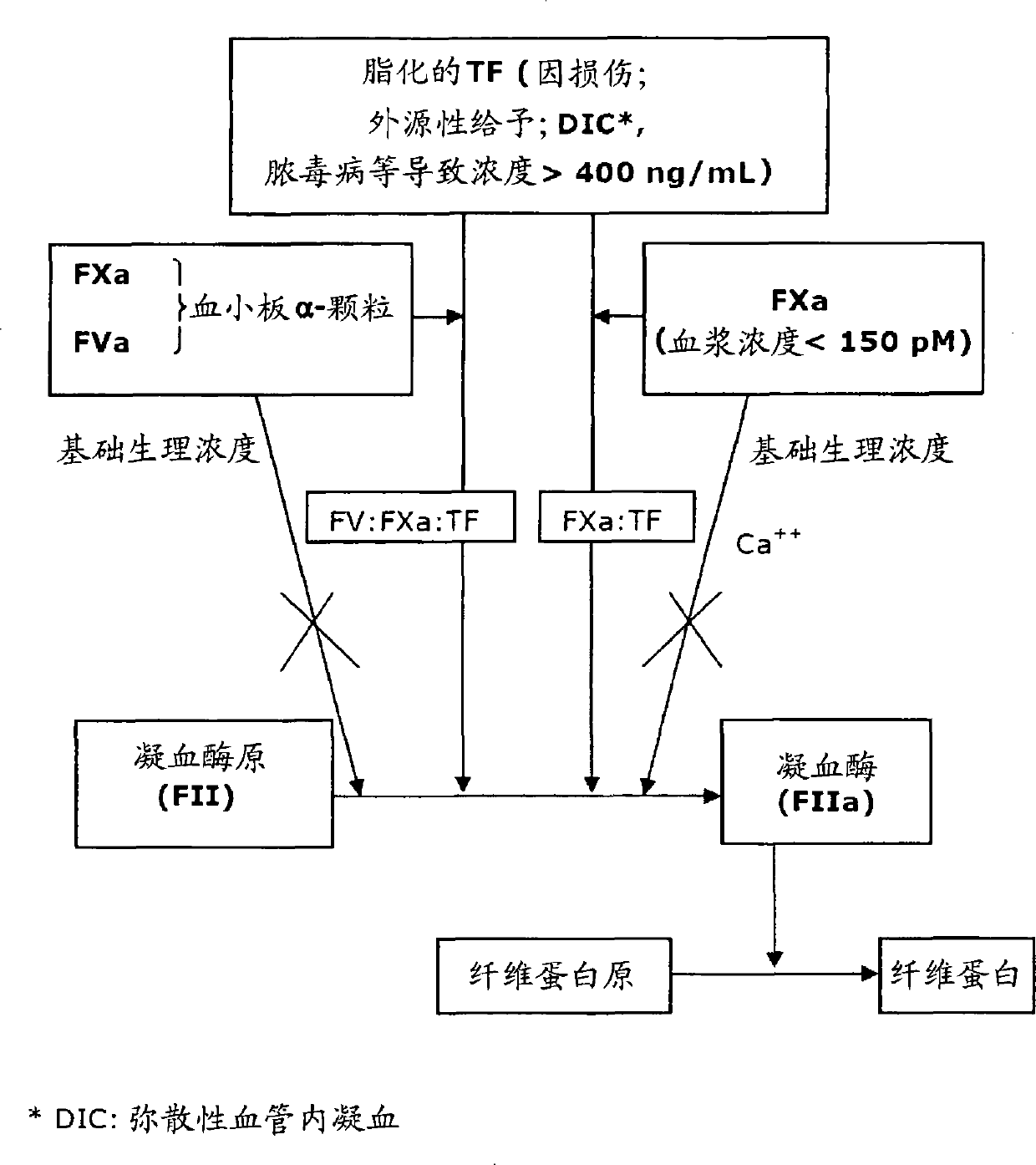
[0462] 1) In the absence of ligand FVII, lipidated TF can directly interact with FXa, thereby significantly increasing its proteolytic activity (amidolysis and thrombin formation activity), and this result shows for the first time a new role of lipidated TF, That is, it is used as a new cofactor of FXa (unrelated to the known FVa).
[0463] 2) Lipidated TF is able to coagulate FVII-deficient plasma. Lipidated TF is therefore a good alternative therapy for such patients (the only existing therapy is expensive human recombinant FVIIa).
[0464] 3) Lipidated TF acts synergistically with NCIS and FXa (at low concentrations that cannot produce coagulation).
Embodiment 2
[0465] The results of Example 2 clearly show:
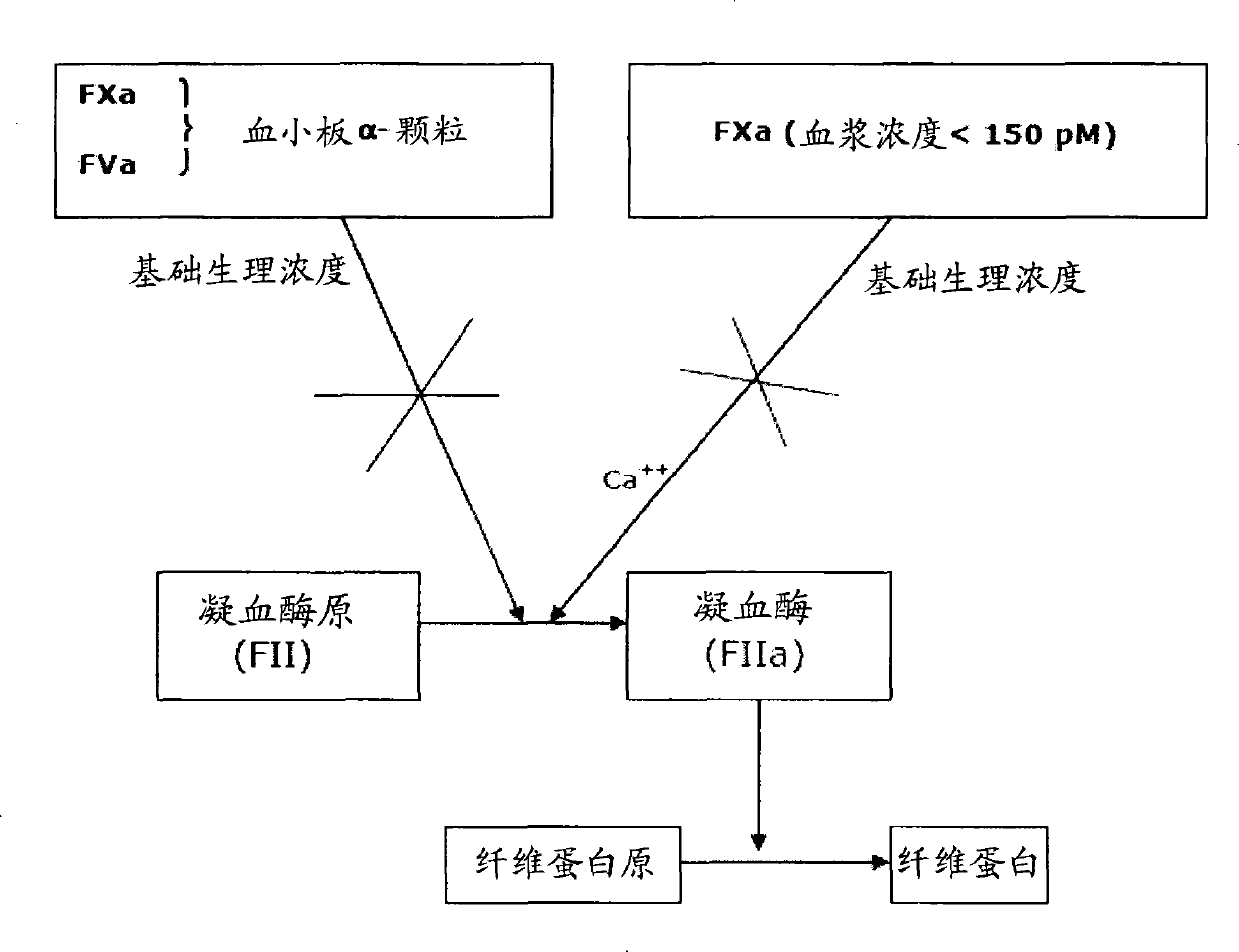
[0466] 1) Lipidated TF enables the hydrolysis of prothrombin by physiological concentration of FXa that cannot produce any procoagulant effect, so that thrombin formation occurs.
[0467] 2) Lipidated TF has a novel role as a cofactor for all proteolytic activities of all forms of FXa (soluble FXa and FXa bound to the prothrombinase complex).
[0468] 3) Even FX-deficient plasma (with traces of FX) can be coagulated by lipidated TF. Therefore, lipidated TF is a good alternative therapy for such patients.
[0469] 4) Lipidated TF synergizes with NCIS in the stimulation of FXa activity.
Embodiment 3
[0470] The results of Example 3 clearly show that:
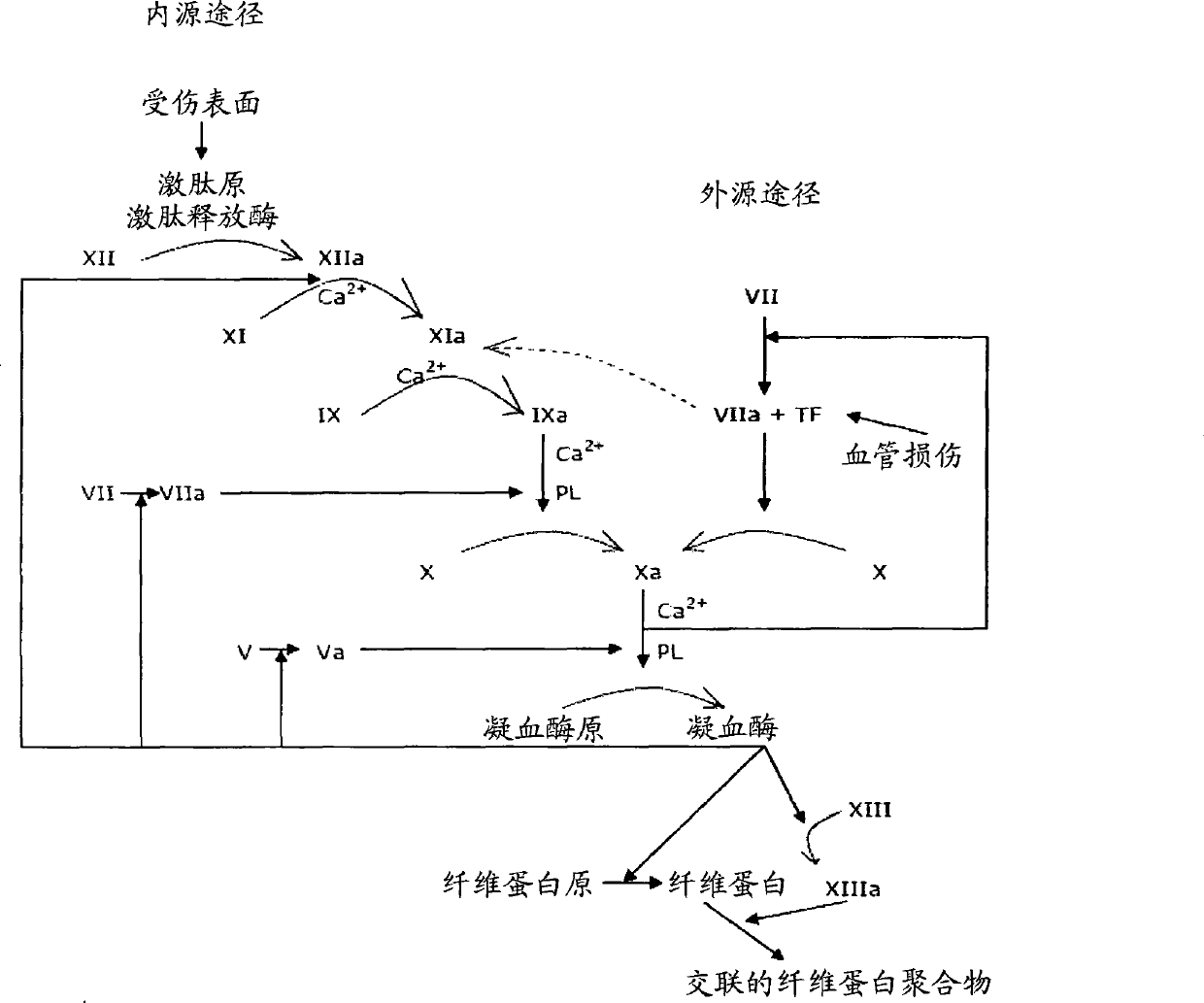
[0471] 1) Lipidated TF can coagulate blood in patients with hemophilia (FVIII, FIX and FXI). Therefore, lipidated TF is a good alternative therapy for such patients.
[0472] 2) Lipidated TF can induce coagulation even in the absence of FV. These results clearly show that lipidated TF has a strong stimulatory effect on FXa, since lipidated TF serves as a cofactor to elicit the same stimulatory effect when its cofactor is absent (FV-deficient plasma).
[0473] 3) Even in FX-deficient plasma (with traces of FX), lipidated TF can induce coagulation. Lipidated TF is therefore an alternative therapy for such patients.
[0474] 4) Lipidated TF can cause coagulation in heparin- and warfarin-treated plasma, which means that lipidated TF interferes with the action of antithrombin III, and possibly through its stimulation of basal concentrations of FXa, which even Can cause coagulation in warfarin-treated plasma.
[0475] 5) Fina...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com