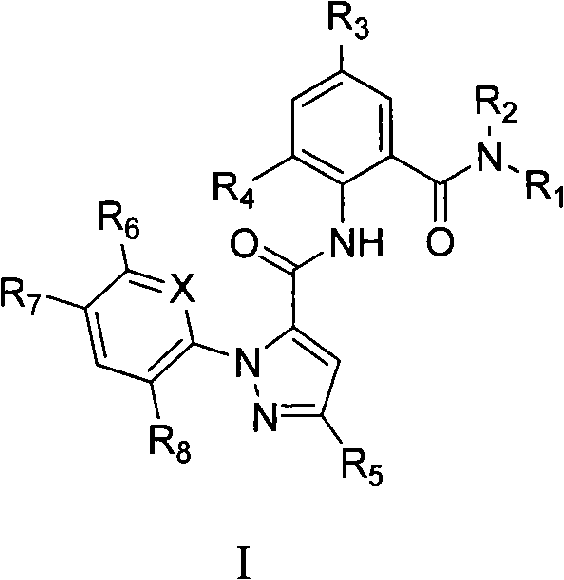
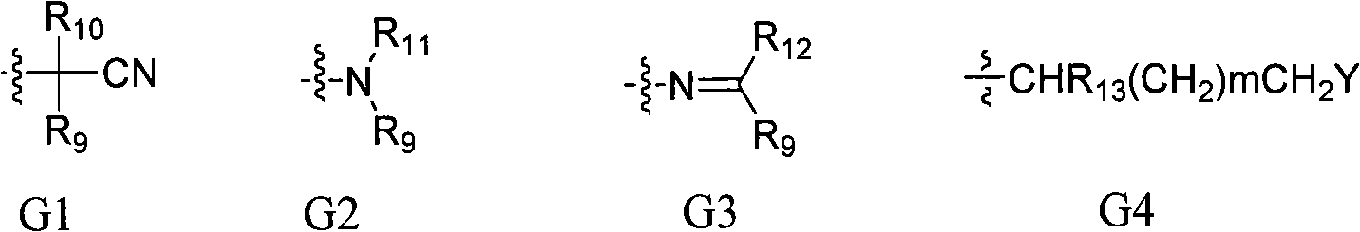
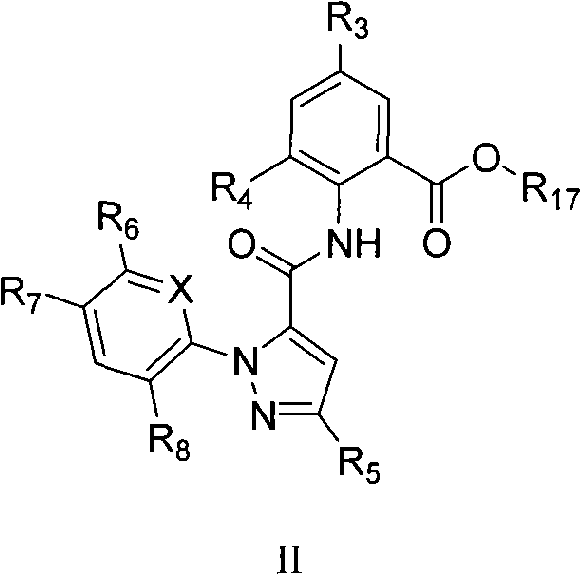
Benzamide compounds and use thereof
A technology of benzamides and compounds, which is applied in the field of benzamides and can solve the problems that the insecticidal activity of benzamides has not been disclosed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0206] Example 1, the preparation of compound 1.3
[0207] (1), the synthesis of 3-methyl-2-nitrobenzoyl chloride
[0208]
[0209] Add 2-nitro-3-methylbenzoic acid (20.0 g, 110 mmol), 100 ml of dichloromethane and oxalyl chloride (21.0 g, 165 mmol) in a 100 ml reaction flask, and then add 5 drops Dimethylformamide, a large amount of gas is evolved. After reacting under stirring at room temperature for 8 hours, the reaction solution was concentrated under reduced pressure, 100 ml of toluene was added, and 22 g of white solid was obtained after concentration under reduced pressure, yield: 100%.
[0210] (2), the synthesis of N-(1-cyanoethyl)-3-methyl-2-nitrobenzamide
[0211]
[0212] Add 2-nitro-3-methylbenzoyl chloride (21.0 grams, 105 mmoles), 200 milliliters of dichloromethane and 2-aminopropionitrile (7.0 grams, 100 mmoles) successively in the reaction bottle of 100 milliliters, Triethylamine (12.0 g, 120 mmol) was added dropwise, and the reaction was stirred at r...
example 2
[0248] Example 2, the preparation of compound 1.9
[0249] (1), 2, the preparation of 4-dicarbonyl pentanoic acid methyl ester
[0250]
[0251] Add 25% methanol solution of sodium methylate (43.2 grams, 0.200 mole), 200 milliliters of methanol in the reaction flask of 1000 milliliters, drop dimethyl oxalate (23.6 grams, 0.200 moles) and acetone (29.2 grams, 0.200 mol) of the mixed solution, the reaction system was maintained at 0 ~ 5 ℃ and stirred for 8 hours. The reaction solution was poured into 200 ml of water, extracted with 150 ml of ethyl acetate, the aqueous layer was adjusted to pH 2-3 with concentrated hydrochloric acid, extracted with 3×200 ml of ethyl acetate, and the organic phase was washed with water and saturated brine. After drying with anhydrous magnesium sulfate and concentrating, 25.9 g of a yellow oil was obtained, and the yield was 90%.
[0252] 1 H NMR (300MHz, CDCl 3 ): 6.396(s, 1H), 3.906(s, 3H), 2.279(s, 3H).
[0253] (2), the preparation of 3...
example 3
[0271] Example 3, the preparation of compound 1.11
[0272]
[0273] Add compound 1.10 (prepared by the operation method of compound 1.9. 0.60 g, 1.12 mmol) and 20 ml of dimethylformamide successively in a 100 ml reaction flask, and dropwise add 28% sodium methylate methanol solution therein under stirring ( 0.24 g, 1.24 mmol), and then slowly heated to 100 ° C for 6 hours. The reaction solution was poured into 50 ml of water, extracted with 3×50 ml of ethyl acetate, the organic phase was washed with saturated brine, dried with anhydrous magnesium sulfate, concentrated under reduced pressure, and the residue was separated by column chromatography (eluent: acetic acid Ethyl ester:petroleum ether=1:6) to obtain 0.22 g of white solid compound 1.11, yield: 37%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com