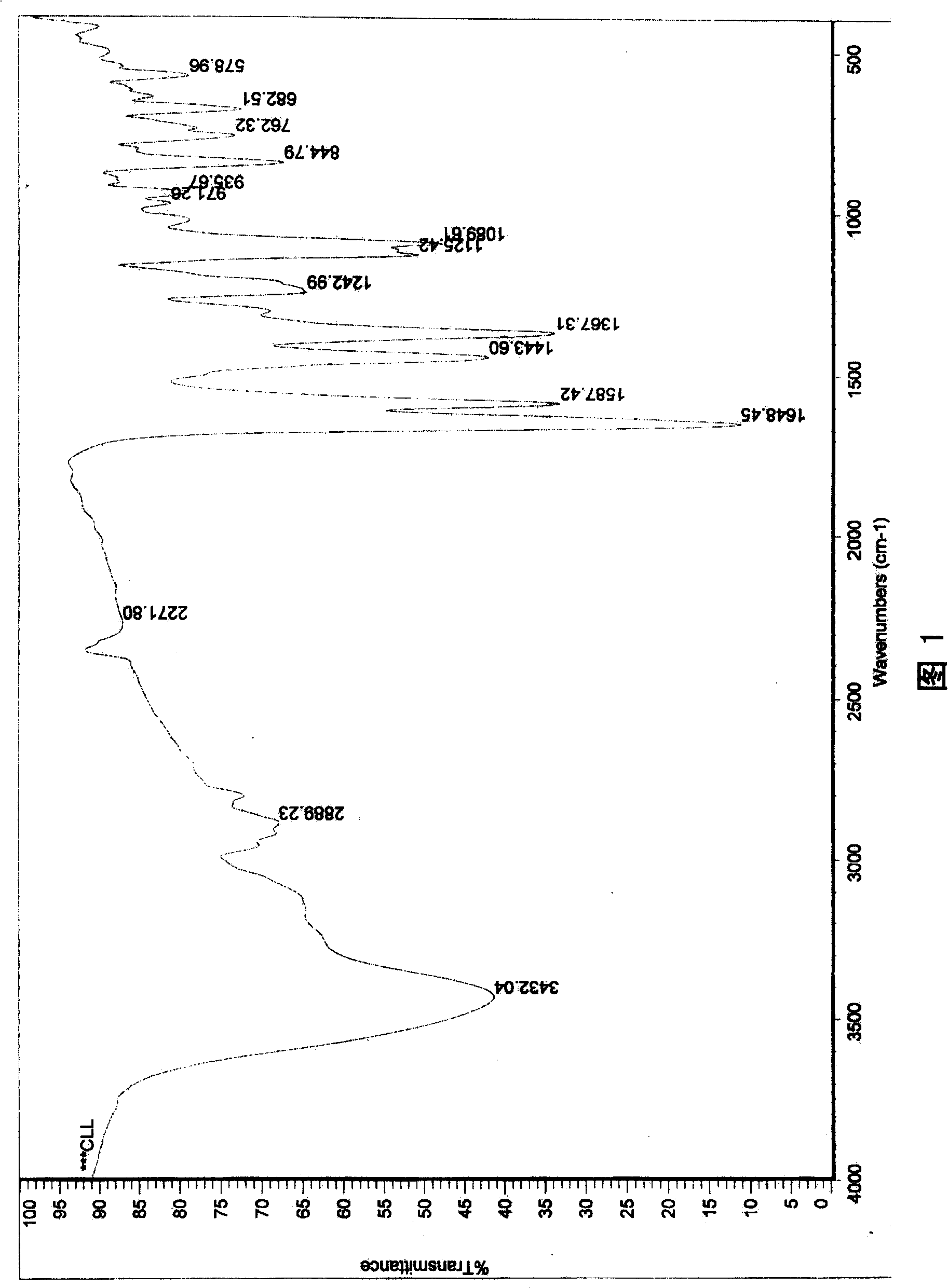
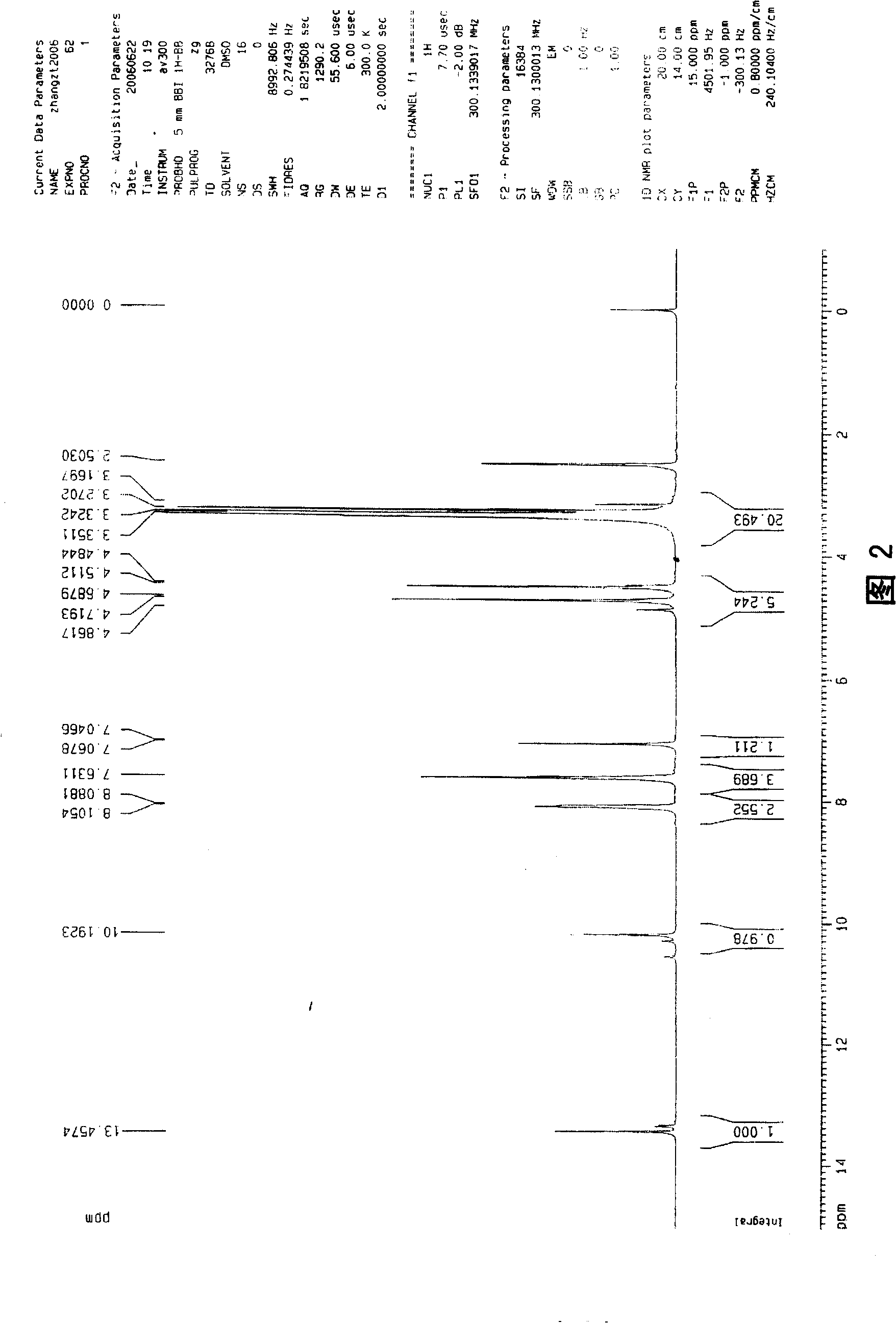
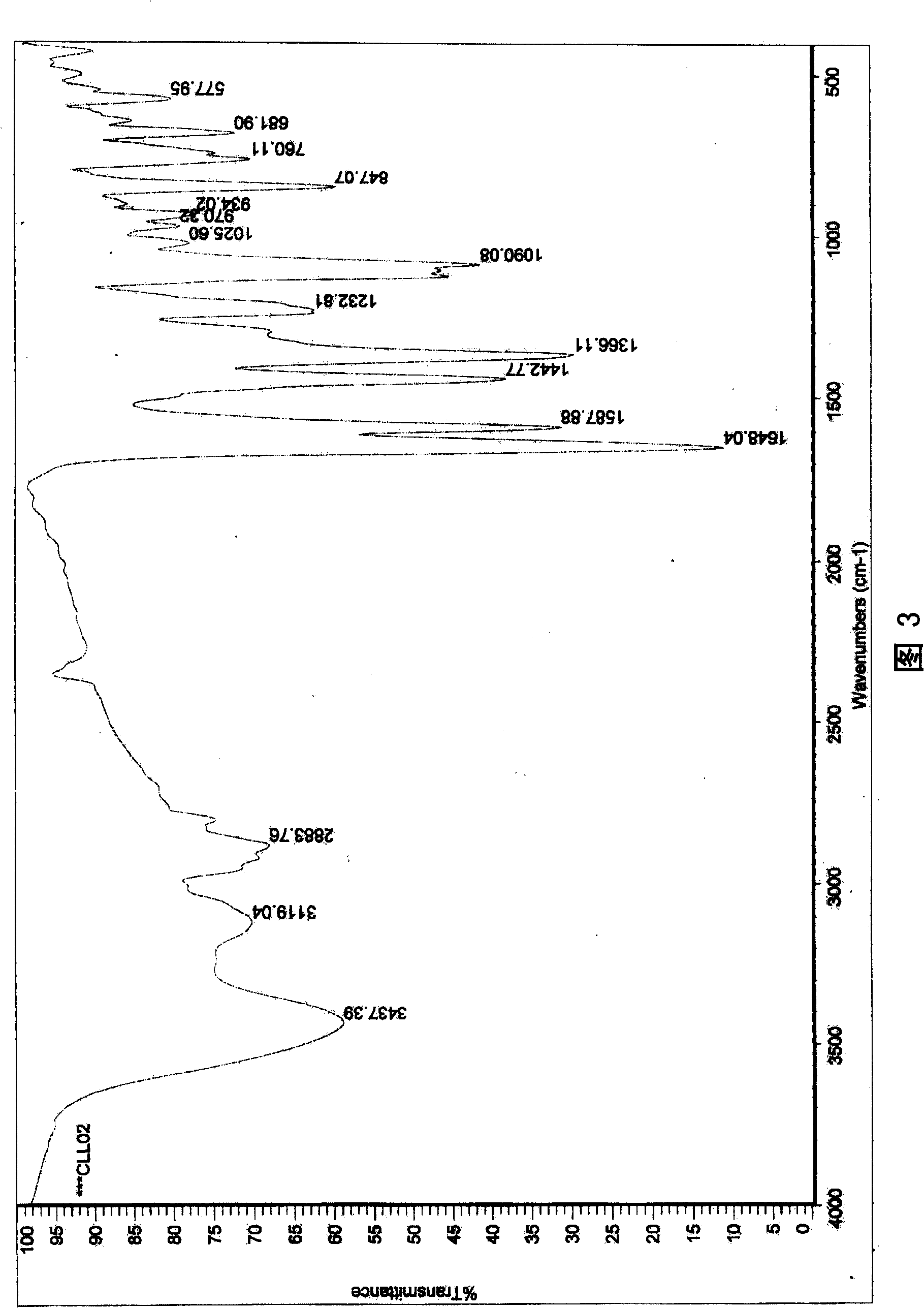
6,8-dimethylol chrysin and 6,8-dimethylol ether chrysin, method for preparing same and pharmaceutical use
A technology of dihydroxyl and chrysin, applied in the direction of drug combination, pharmaceutical formula, medical preparations containing active ingredients, etc., can solve the problem of weak effect, achieve low production cost, good anti-hypoxia effect, and good therapeutic effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] In this example, chrysin, solid KOH equal in mass to chrysin, and methanol solvent 20 times the mass of chrysin were added to the reaction kettle, and chrysin was completely dissolved in the solvent under stirring, and then 37% formaldehyde aqueous solution was added , make chrysin and formaldehyde carry out chemical reaction, the molar ratio of chrysin and formaldehyde used is 1: 5, make the temperature of reaction liquid be 15 ℃ with temperature adjustment device, after reacting for 40 hours, add 10% HCl to acidify to pH = 4. Poured into 5 times the volume of the reaction solution in water, a pale yellow precipitate precipitated out, filtered to obtain the crude product of formula (1), and purified the crude product by recrystallization with methanol to obtain the pure product of formula (1) (yield 85%). Adopt the formula (1) prepared by the present embodiment, after testing, its physical and chemical properties are as follows:
[0053] Pale yellow needle-like crystal...
Embodiment 2
[0059] In this example, add chrysin, solid KOH of the same quality as chrysin, and methanol solvent with 15 times the mass of chrysin in the reaction kettle, and completely dissolve chrysin in the solvent under stirring, then add paraformaldehyde to make Chrysin and paraformaldehyde carry out chemical reaction, the molar ratio of chrysin and paraformaldehyde used is 1: 3, make the temperature of reaction solution be 50 ℃ with temperature regulating device, after reacting for 10 hours, add 5% HCl to acidify to pH = 5, poured into 7 times the volume of the reaction solution in water, a pale yellow precipitate precipitated out, and the other processes were the same as in Example 1.
Embodiment 3
[0061] In this example, add chrysin, solid KOH equal in mass to chrysin, and methanol solvent with 25 times the mass of chrysin in the reaction kettle, completely dissolve chrysin in the solvent under stirring, and then add 15% formaldehyde solution , make chrysin and formaldehyde carry out chemical reaction, the molar ratio of chrysin and formaldehyde used is 1: 9, make the temperature of reaction liquid be 5 ℃ with temperature regulating device, after reacting for 70 hours, add 15% HCl to acidify to pH = 3. Poured into 5 times the volume of the reaction solution in water, there was a pale yellow precipitate, and the other processes were the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com