Nitrogen-oxygen free radical compound with anti-hypoxia injury activity and preparation and application thereof
A nitroxide free radical, anti-hypoxia technology, applied in organic chemistry, anti-toxic agent, drug combination, etc., can solve the problems of increased generation of free radicals, decompensation of antioxidant system, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
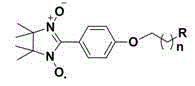
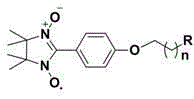
[0053] Embodiment 1, the synthesis and structural characterization of compound 1
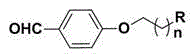
[0054] (1) Synthesis of 4-(2-bromoethoxy)benzaldehyde: 1.22 g (10 mmol) 4-hydroxybenzaldehyde, 2.07 g (15 mmol) K 2 CO 3 and 2.82 g (15 mmol) of 1,2-dibromoethane were dissolved in 50 mL of acetone, refluxed for 8 h, and the reaction was stopped. After cooling to room temperature, the acetone was removed under reduced pressure, and 100 mL of water was added. 2 Cl 2 Extraction (50 mL × 3), combined organic phase, anhydrous Na 2 SO 4 Dry overnight, filter, remove the solvent under reduced pressure and separate by flash column chromatography to obtain 4-(2-bromoethoxy)benzaldehyde. White solid 1.83 g, yield 80%. 1 H-NMR (CDCl 3 , 400 MHz) δ : 9.89(s, 1H), 7.85(d, J =8.8 Hz, 2H), 7.02 (d, J =8.4 Hz, 2H), 4.38 (t, J =6.0 Hz, 2H), 3.68 (t, J = 6.0 Hz, 2H). ESI-MS (m / z): 230 [M+H] + .
[0055] (2) Synthesis of 4-(2-(dimethylamino)ethoxy)benzaldehyde: 2.29 g (10 mmol) 4-(2-bromoethoxy)benz...
Embodiment 2
[0058] Embodiment 2, the synthesis and structural characterization of compound 2
[0059] (1) Synthesis of 4-(2-bromoethoxy)benzaldehyde: same as Example 1.
[0060] (2) Synthesis of 4-(2-(diethylamino)ethoxy)benzaldehyde: 2.29 g (10 mmol) 4-(2-bromoethoxy)benzaldehyde, 2.07 g (15 mmol) K 2 CO 3 and 15 mmol of diethylamine were dissolved in 50 mL of acetonitrile, and refluxed for 11 h. After the reaction was complete as monitored by TLC, the reaction system was cooled to room temperature, filtered, and the filter cake was washed with a small amount of acetonitrile. After removing the acetonitrile under reduced pressure, flash column chromatography Separation gave 4-(2-(diethylamino)ethoxy)benzaldehyde. 1.50 g of light yellow oily matter, yield 68%. 1 H-NMR (CDCl 3 , 400 MHz) δ : 9.88(s, 1H), 7.83(d, J =8.8 Hz, 2H), 7.01 (d, J =8.4 Hz, 2H), 4.13(t, J =6.0 Hz, 2H), 2.90 (t, J=6.0 Hz, 2H) 2.68~2.62 (m, 4H), 1.08 (t, J =6.8Hz, 6H). 13 C-NMR (CDCl 3 , 100 MHz) δ : 190...
Embodiment 3
[0063] Example 3: Synthesis and structural characterization of compound 3
[0064] (1) Synthesis of 4-(2-bromoethoxy)benzaldehyde: same as Example 1.
[0065] (2) Synthesis of 4-(2-(tetrahydropyrrolyl)ethoxy)benzaldehyde: 2.29 g (10 mmol) 4-(2-bromoethoxy)benzaldehyde, 2.07 g (15 mmol) K 2 CO 3 and 15 mmol tetrahydropyrrole were dissolved in 50 mL acetonitrile, and refluxed for 17 h. After the reaction was complete as monitored by TLC, the reaction system was cooled to room temperature, filtered, and the filter cake was washed with a small amount of acetonitrile. After removing the acetonitrile under reduced pressure, flash column chromatography After separation, 1.35 g of light yellow oil was obtained, with a yield of 62%. 1 H-NMR (CDCl 3 , 400 MHz) δ : 9.88(s, 1H), 7.83(d, J =8.4 Hz, 2H), 7.02 (d, J =8.8 Hz, 2H), 4.21(t, J =5.6 Hz, 2H), 2.95 (t, J =6.0 Hz, 2H), 2.65 (s, 4H), 1.86 (s, 4H). 13 C-NMR (CDCl 3 , 100 MHz) δ : 190.7, 163.7, 131.9, 129.9, 114.8, 67.3, 54....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com