Culture collection process
A kind of strain preservation and strain preservation technology, applied in the field of strain preservation, can solve the problems of people who have no relevant knowledge, such as illness, insolvability, and environmental pollution, and achieve the effects of prolonging the preservation time, improving production efficiency, and reducing damage
Inactive Publication Date: 2008-11-19
药大制药有限公司
View PDF0 Cites 13 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
This method is very wasteful, and the disposal of the waste after the use of the same strains must be very careful. If you are not careful, it will pollute the environment, and even cause people without relevant knowledge to get sick, and the consequences are very serious.
At the same time, there are few relevant documents and materials at home and abroad, which cannot solve the current problems
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment Construction
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
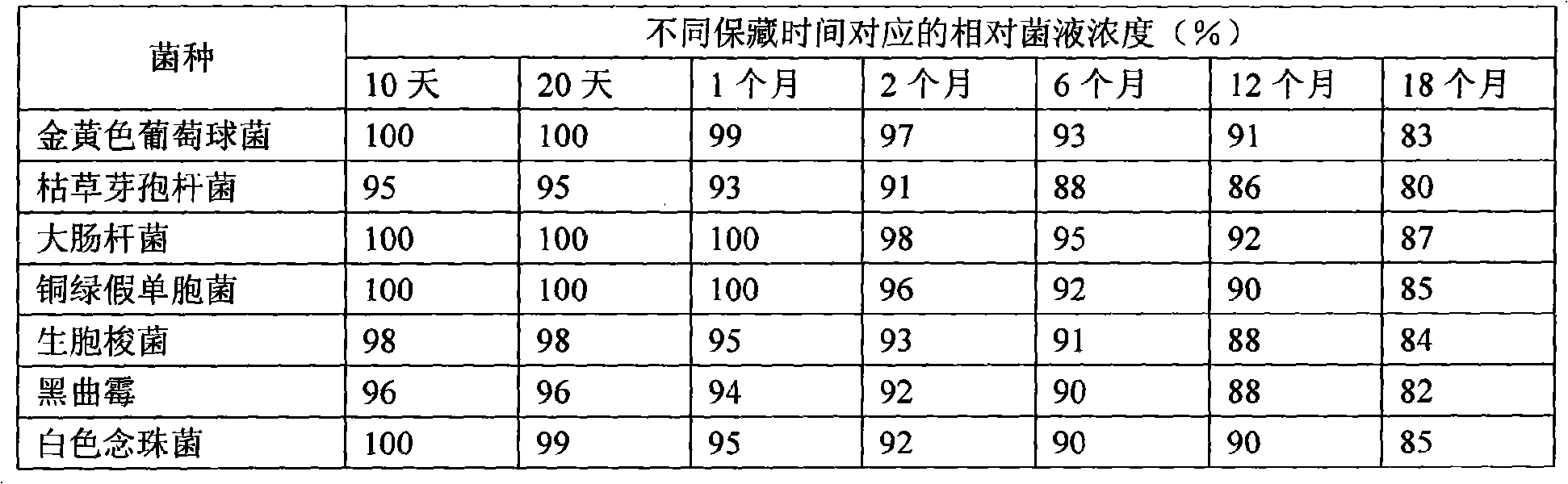
The invention provides a spawn preservation method adopting semisolid low-temperature air-tight culture. A spawn is inoculated in an aseptic semisolid culture medium which is vertically stored at an ambient temperature of between 6 and 8 DEG C after aseptic liquid paraffin is added into the culture medium. The spawn is staphylococcus aureus, escherichia coli, pseudomonas aeruginosa, bacillus subtilis, clostridium orogenes, aspergillus niger or candida albicans. The specific process is as follows: preparing the semisolid culture medium, carrying out sub-package and sterilization, and then culturing for 2 to 3 days, inoculating the spawn into the semisolid culture medium in a mode of stab inoculation after asepsis is ensured, with the number of stab inoculation points parallel with the plane of the culture medium being no less than 3; pouring the aseptic liquid paraffin with a height of 1 to 2 cm into the culture medium which is sealed by a sealing film and is stored in a refrigerator at a temperature of between 6 and 8 DEG C. The method can not only prolong the spawn preservation time but also keep a relatively stable spawn concentration, thereby saving much cost for production and test, reducing the treatment and discharge of rejected material after spawn use, and reducing environmental hazard.
Description
technical field The invention relates to a strain preservation method. Background technique According to the "Pharmacopoeia of the People's Republic of China", the use of bacterial strains in the microbial limit test and sterility test of drugs is a must for positive controls. Required in validation. Therefore, the use and preservation of strains has always been the focus of microbiology laboratories for a long time. There are 7 strains commonly used, namely: Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis, Clostridium sporogenes, Aspergillus niger, and Streptococcus albicans. At present, domestic drug manufacturers have been using the strain preservation technology many years ago, because this method has been used for many years and is relatively stable and reliable. This method is as follows: the bacterial strains purchased from the drug inspection institute and stored on a low-temperature inclined plane are used normally. The purchas...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C12N1/04C12R1/445C12R1/19C12R1/385C12R1/125C12R1/01C12R1/685C12R1/725
Inventor 王卉刘明越蔡振利
Owner 药大制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com