High-temperature fuel cell system having anode loading functional coating with methane as main fuel
A fuel cell system, battery system technology, applied in the direction of fuel cells, fuel cell parts, solid electrolyte fuel cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
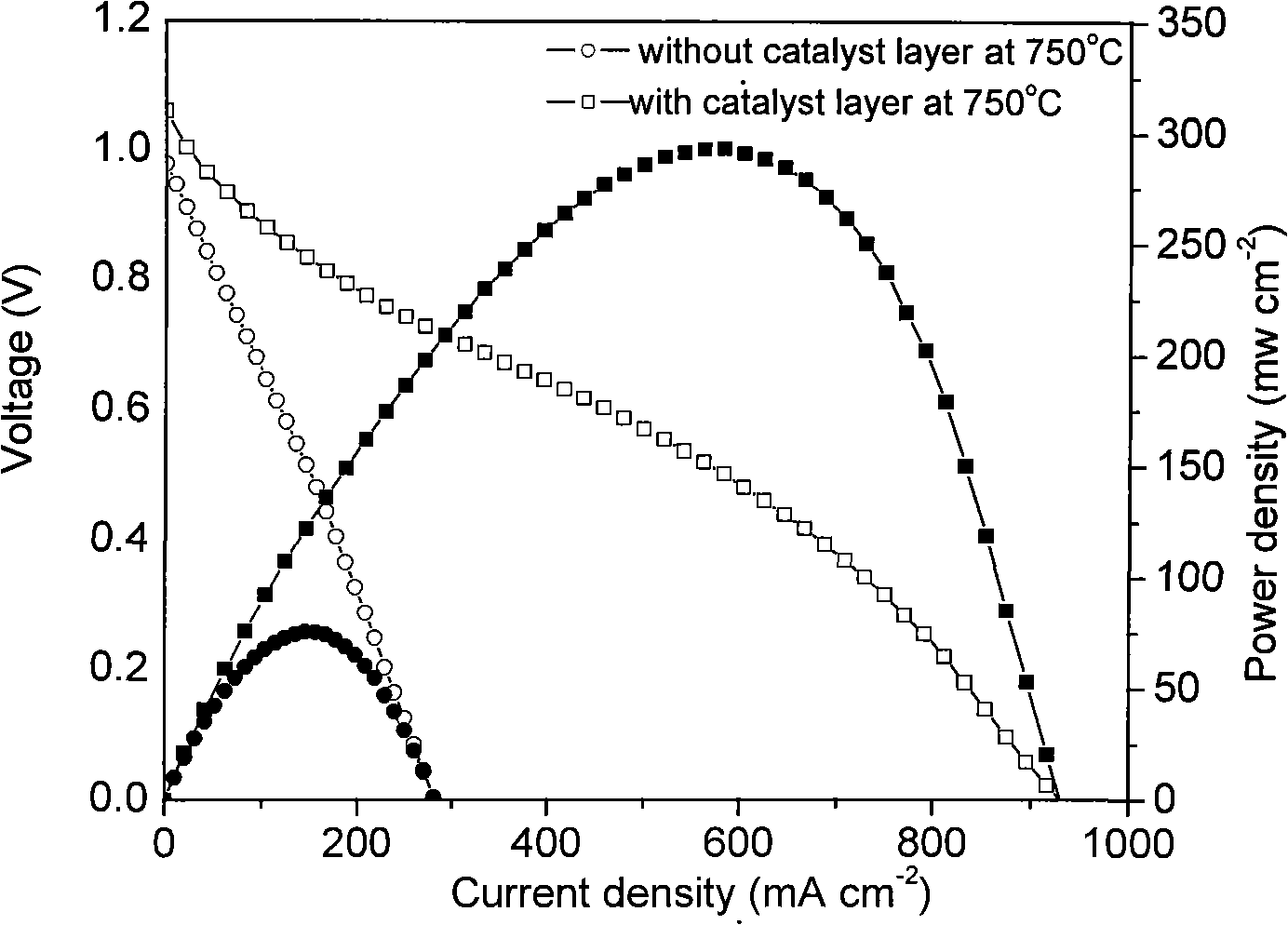
[0031] Example 1: Ni+ScSZ|ScSZ|La with methane and oxygen as fuel 0.8 Sr 0.2 MnO 3 fuel cell system
[0032] First, prepare the catalyst: weigh Ni(NO 3 ) 2 ·6H 2 O 3.468g, Al(NO 3 ) 2 9H 2 O 68.43g. Dissolve with deionized water, add glycine, wherein, the molar ratio of glycine to total metal ions (G / M n+ ) ratio of 2:1, after being completely dissolved, the solution was heated to a viscous state at 80°C with stirring, and then spontaneously burned in an oven at a temperature of 240°C. After cooling, it is calcined at 800°C for 5 hours in a muffle furnace, and ground into powder to obtain NiO-Al 2 o 3 Powder.
[0033] Then, prepare the battery: weigh 30g of ScSZ powder and 45g of NiO powder, add 5.25g of PVB and an appropriate amount of ethanol, take it out after ball milling for 24 hours, maintain 80°C for drying, and then ball mill for 40 minutes to obtain the required anode composite powder. Weigh 0.3g of anode powder and 0.02g of ScSZ to make a green body cont...
Embodiment 2
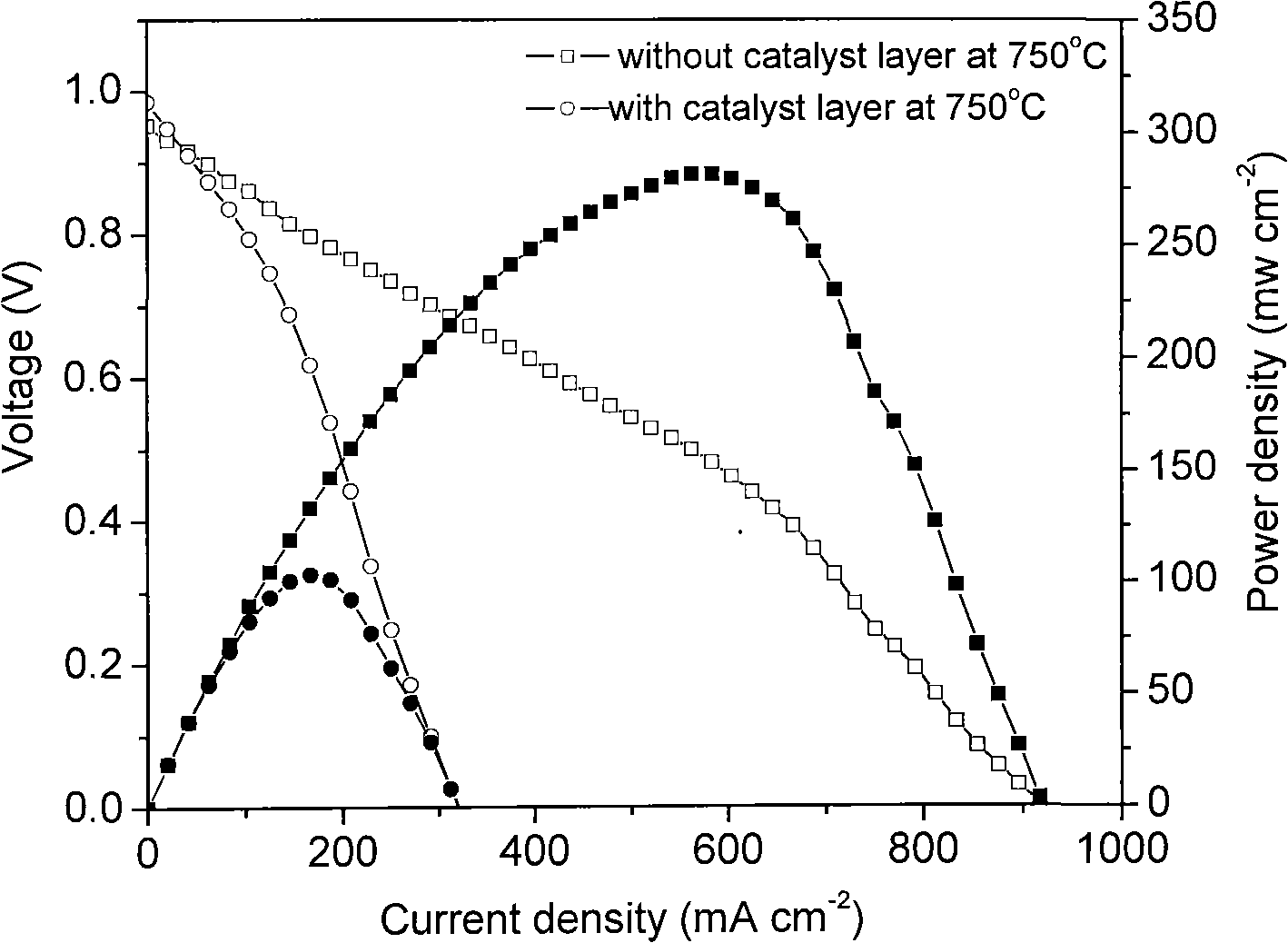
[0036] Example 2: Ni+ScSz|ScSz|La with methane and steam as fuel 0.8 Sr 0.2 MnO 3 fuel cell system.
[0037] First, prepare the catalyst: weigh Ni(NO 3 ) 2 ·6H 2 O 3.964g, Al(NO 3 ) 2 · 9H 2 O 67.70g. Dissolve with deionized water, add glycine, wherein, the molar ratio of glycine to total metal ions (G / M n+ ) ratio of 2.5:1. After being completely dissolved, the solution was heated at 100°C under stirring to become viscous, and then spontaneously burned in an oven at a temperature of 230°C. After cooling, it is calcined at 850°C for 6 hours in a muffle furnace, and ground into powder to obtain NiO-Al 2 o 3 Powder.
[0038] The method for preparing the battery is the same as in Example 1.
[0039] Secondly, prepare the anode catalytic coating, 1gNiO-Al 2 o 3 Add 18ml of isopropanol, 1.0ml of glycerin, and 3.5ml of ethylene glycol to the powder, put it into the high-energy ball mill for 40 minutes, take it out, spray it on the surface of the anode with a spray gun...
Embodiment 3
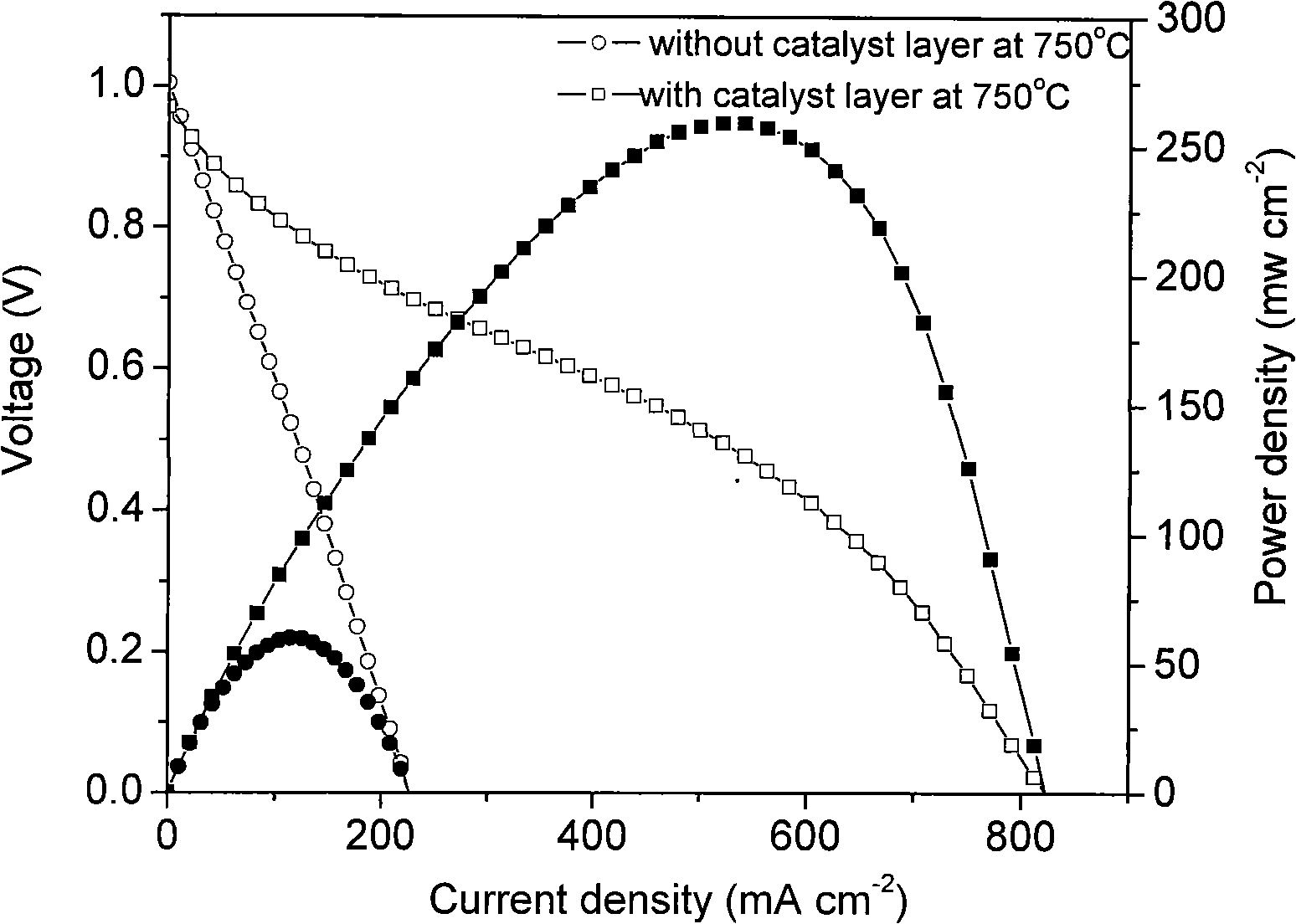
[0041] Example 3: Ni+ScSZ|ScSZ|La with methane and carbon dioxide as fuel 0.8 Sr 0.2 MnO 3 fuel cell system
[0042] First, prepare the catalyst: weigh Ru(NO 3 ) 3 1.988g, Ce(NO 3 ) 4 ·6H 2 O 23.46g. Dissolve with deionized water, add glycine, wherein, the molar ratio of glycine to total metal ions (G / M n+ ) ratio of 3:1. After being completely dissolved, the solution was heated to a viscous state at 110°C with stirring, and then spontaneously burned in an oven at a temperature of 200°C. After cooling, it was calcined at 800°C for 7 hours in a muffle furnace, and ground into powder to obtain RuO 2 -CeO 2 Powder.
[0043] The battery was prepared, and the catalyst coating method was the same as in Example 1.
[0044] Then, a solid oxide fuel cell test using methane and carbon dioxide (2:1) as fuel was carried out, a four-probe structure was adopted, and a casing was added outside the reactor. From image 3 It can be seen from the figure that when no catalytic laye...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Power density | aaaaa | aaaaa |
| Power density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com