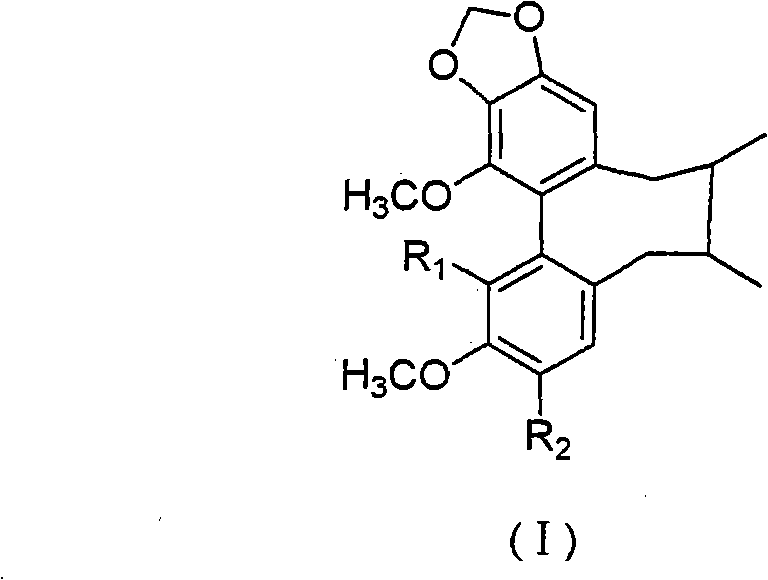
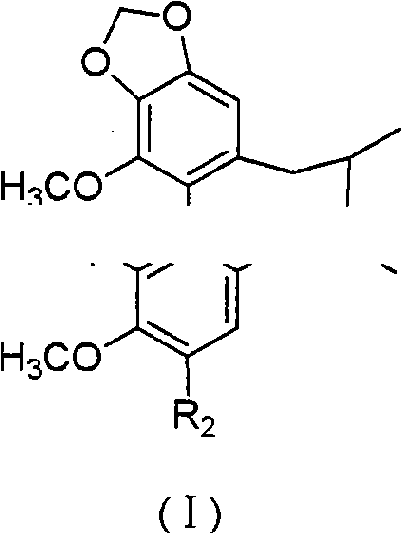
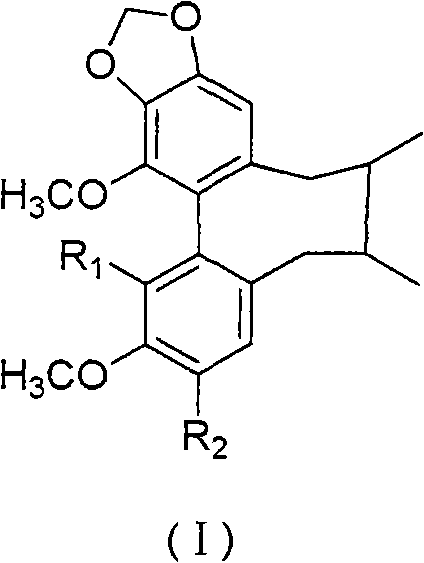
Preparation method for couplet benzene ring octadiene system lignans and application thereof
A technology of biphenyl cyclooctadiene and lignans, which is applied in the fields of heterocyclic compound active ingredients, antiviral agents, organic chemistry, etc., and can solve the synthesis method of biphenyl cyclooctadiene lignin compounds that have not been seen reports and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090]
[0091] Dissolve 0.1 mol of gallic acid in 250 mL of freshly distilled methanol, slowly add 0.01 mol of concentrated sulfuric acid dropwise, and reflux for 17 hours. After the completion of the reaction detected by TLC, part of the solvent was evaporated under reduced pressure, and poured into 500 mL of saturated potassium carbonate aqueous solution, a white solid was precipitated. A white solid was separated and the aqueous phase was extracted with ethyl acetate (3 x 200 mL). The organic phases were combined, dried over anhydrous sodium sulfate, and concentrated, and the obtained white solid was compound 1 after drying.
[0092] The yield of this reaction process was 97%. The relevant test data is as follows:
[0093] 1 H-NMR (300MHz, CDCl 3 ): δ=6.82(2H), 3.87(3H).
Embodiment 2
[0095]
[0096] Equipped with a Dean-Stark apparatus, take 150 mmol of the compound obtained in Example 1 and dissolve it in 200 mL of benzene, add 150 mmol of triethyl orthoformate and 5 mg / mmol of acidic resin Amberlyst 15E, reflux, filter after TLC detection and trace the disappearance of raw materials, and concentrate the filtrate Compound 2 was obtained by column chromatography.
[0097] The yield of this reaction process was 98%. The relevant test data is as follows:
[0098] 1 H-NMR (300MHz, CDCl 3 ): δ=7.39(1H), 7.28(1H), 7.06(1H), 3.87(3H), 3.56(2H), 1.2(3H).
Embodiment 3
[0100]
[0101] Dissolve 240mmol of the compound obtained in Example 2 in 200mL of acetone, add 60mmol of methyl iodide or benzyl bromide, and 60mmol of potassium carbonate, reflux reaction, TLC detection after the reaction is complete, filter, concentrate, and column chromatography to obtain compound 3 or compound 4.
[0102] The yield of this reaction process was 96%. The relevant test data is as follows:
[0103] Compound 3 1 H-NMR (300MHz, CDCl 3 ): δ=7.28(1H), 7.25(1H), 7.10(1H), 3.89(3H), 3.86(3H), 3.62(2H), 1.34(3H).
[0104] Compound 4 1 H-NMR (300MHz, CDCl 3 ): δ=7.21-7.41(8H), 5.06(2H), 3.87(3H), 3.81(2H), 1.23(3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com