Arsenic trioxide solid lipid nano granule and formulation
A technology of solid lipid nanometer and arsenic trioxide, which is applied in the directions of liposome delivery, liquid delivery, medical preparations of inactive ingredients, etc., can solve problems such as poor solubility and stability, improve compliance, solve stability problems, The effect of reducing medication costs and risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The prescription consists of:
[0051]
[0052] Preparation method: Heating glyceryl tristearate and lecithin in a water bath at 40-100°C until melting, adding arsenic trioxide pulverized by ball milling, and dispersing evenly; rapidly cooling the drug-containing melt with dry ice or liquid nitrogen, passing through ball milling or milk Bowl milling grinds solid drug-containing lipids into particles; dissolves Poloxamer 188 and EDTA-2Na in distilled water to make a water phase, and then disperses the solid lipid particles into a low-temperature water phase to form primary Suspension: Finally, the primary suspension is subjected to high-pressure homogenization at room temperature or below room temperature to obtain a suspension that can be used to prepare the final product.
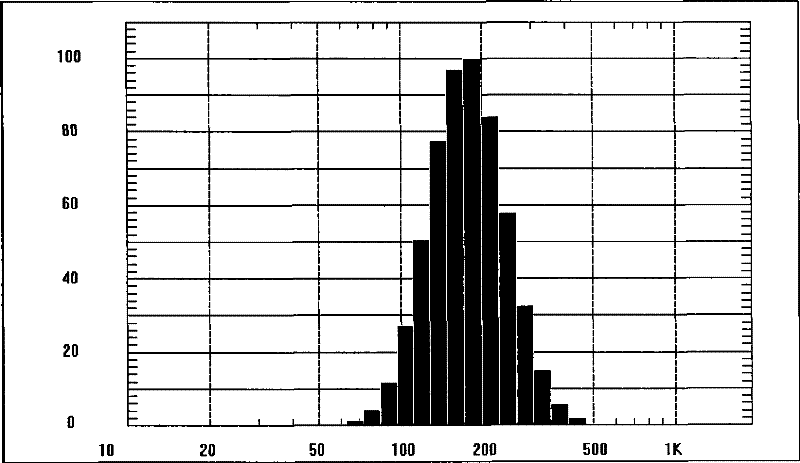
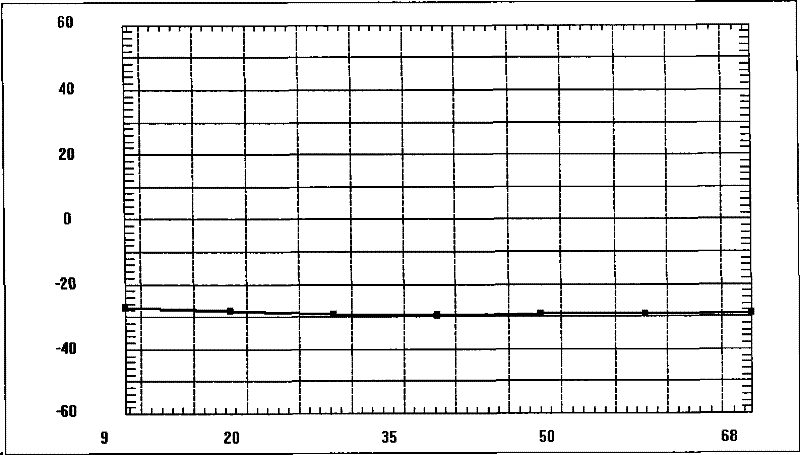
[0053] Check: the encapsulation rate of nanoparticles is 93.4%, the average particle size of nanoparticles is 190nm, see the attached figure 1 , Zeta potential -29mV, see attached figure 2 . ...
Embodiment 2
[0055] The prescription consists of:
[0056] Preparation method: heat glyceryl tristearate, glyceryl distearate, and lecithin in a water bath at 40-100°C until melting, add arsenic trioxide pulverized by ball milling, and disperse evenly; mix evenly as the oil phase; Mu 188 and EDTA-2Na are fully dispersed in water, heated to the same temperature as the oil phase as the water phase; add the oil phase to the water phase under high-speed stirring, and continue to stir for a certain period of time to form colostrum; high-pressure homogenization for 2 to 10 times; Cool to room temperature to obtain a suspension that can be used to prepare the final product.
[0057] Inspection: the encapsulation efficiency of the nanoparticles is 91.5%, the average particle size of the nanoparticles is 200nm, and the Zeta potential is -29mV.
Embodiment 3
[0059] The prescription consists of:
[0060] Preparation method: heat stearic acid, glyceryl palmitate, and lecithin in a water bath at 40-100°C to melt, add arsenic trioxide pulverized by ball milling, and mix uniformly as an oil phase; poloxamer 188, Tween-80, Dissolve glycerin and EDTA-2Na in water, heat to the same temperature as the oil phase as the water phase; add the oil phase to the water phase under stirring, and stir gently to form a transparent nanoemulsion, and then disperse the hot nanoemulsion in In cold water (2-3°C), a suspension that can be used to prepare the final product is obtained. Inspection: the encapsulation efficiency of the nanoparticles is 90.8%, the average particle size of the nanoparticles is 162nm, and the Zeta potential is -31mV.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com