Method for preparing dichlorohydrin by glycerol hydrochlorination
A technology of dichloropropanol and hydrochlorination, applied in the direction of introducing halogen preparation, organic chemistry, etc., can solve the problems of high process energy consumption, difficult scale-up production, low utilization rate of equipment, etc., and achieve simple operation control and easy scale-up production , The effect of small investment in equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
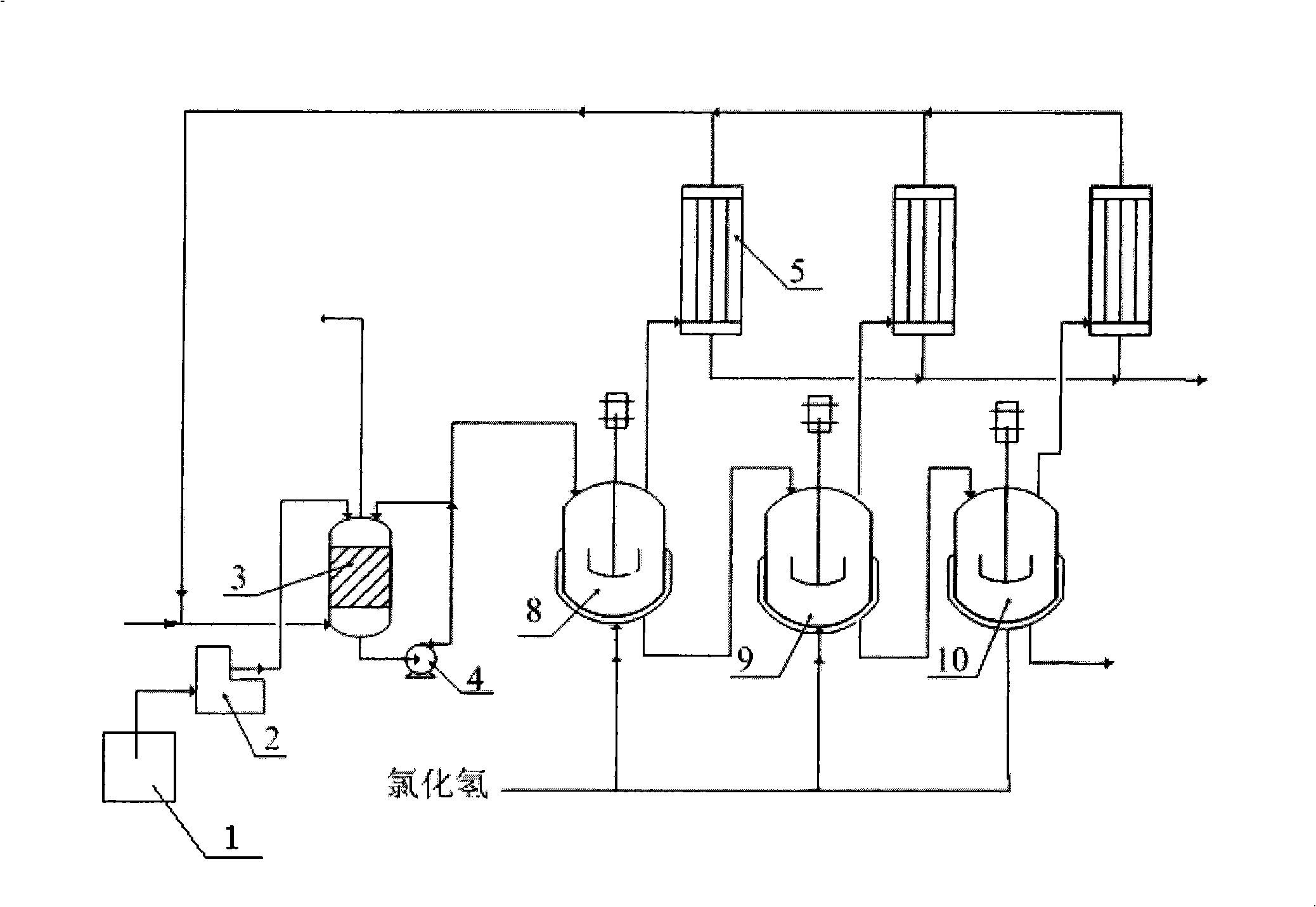
[0044] use figure 1 The process uses 3 stirred reactors.
[0045] The process conditions and operation process are as follows:
[0046] The gas containing hydrogen chloride is produced by the cracking of dichloroethane, and the weight content is 99.5%;
[0047] Catalyst is propionic acid, and consumption is 5% of glycerol weight;
[0048] Propionic acid and glycerol (98% secondary product) are mixed in the storage tank 1, and pumped into the absorption reaction tower shown at a rate of 6.0kg / h through the metering pump 2, and at the same time, the gas containing hydrogen chloride is sent into the absorption reaction tower from the bottom. Reaction tower 3, the flow rate is 0.5m 3 / h;
[0049] The material part at the bottom of the absorption reaction tower 3 is sent back to the top of the absorption reaction tower 3 through the liquid phase circulation pump 4, and the part is divided into the first stirred tank 8;
[0050]Then the reaction solution enters the second stirr...
Embodiment 2
[0057] use figure 1 The process uses 3 stirred reactors.
[0058] The process conditions are as follows:
[0059] Catalyst is propionic acid, and consumption is 5% of glycerol weight;
[0060] The gas containing hydrogen chloride is produced by the cracking of dichloroethane, and the weight content is 99.5%;
[0061] After the catalyst and glycerin (98% industrial product) are mixed in the storage tank 1, they are pumped into the absorption reaction tower shown at a rate of 10.0kg / h through the metering pump 2, and at the same time, the gas containing hydrogen chloride is sent into the absorption reaction tower from the bottom 3. The flow rate is 1.1m 3 / h;
[0062] The weight ratio of the material sent back to the top of the absorption reaction tower 3 to the first stirred tank 8 is 100:1;
[0063] The temperatures of the absorption reaction tower 3 and the stirred tank are respectively controlled at 90°C, 105°C, 110°C and 110°C;
[0064] The residence time of the react...
Embodiment 3
[0068] use figure 1 The process uses 3 stirred reactors.
[0069] The process conditions are as follows:
[0070] Catalyst is adipic acid, and consumption is 10% of glycerin weight;
[0071] The gas containing hydrogen chloride is hydrogen chloride gas;
[0072] After the catalyst and glycerin are mixed in the storage tank 1, they are pumped into the absorption reaction tower shown by the metering pump 2 at a rate of 6.0kg / h. At the same time, the gas containing hydrogen chloride is sent into the absorption reaction tower 3 from the bottom with a flow rate of 0.4m 3 / h;
[0073] The weight ratio of the material sent back to the top of the absorption reaction tower 3 to the first stirred tank 8 is 150:1;
[0074] The temperatures of the absorption reaction tower 3 and the stirred tank are respectively controlled at 90°C, 90°C, 110°C and 130°C;
[0075] Other conditions are the same as example 1. After the reaction is stable, the weight flow rate and component content of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com