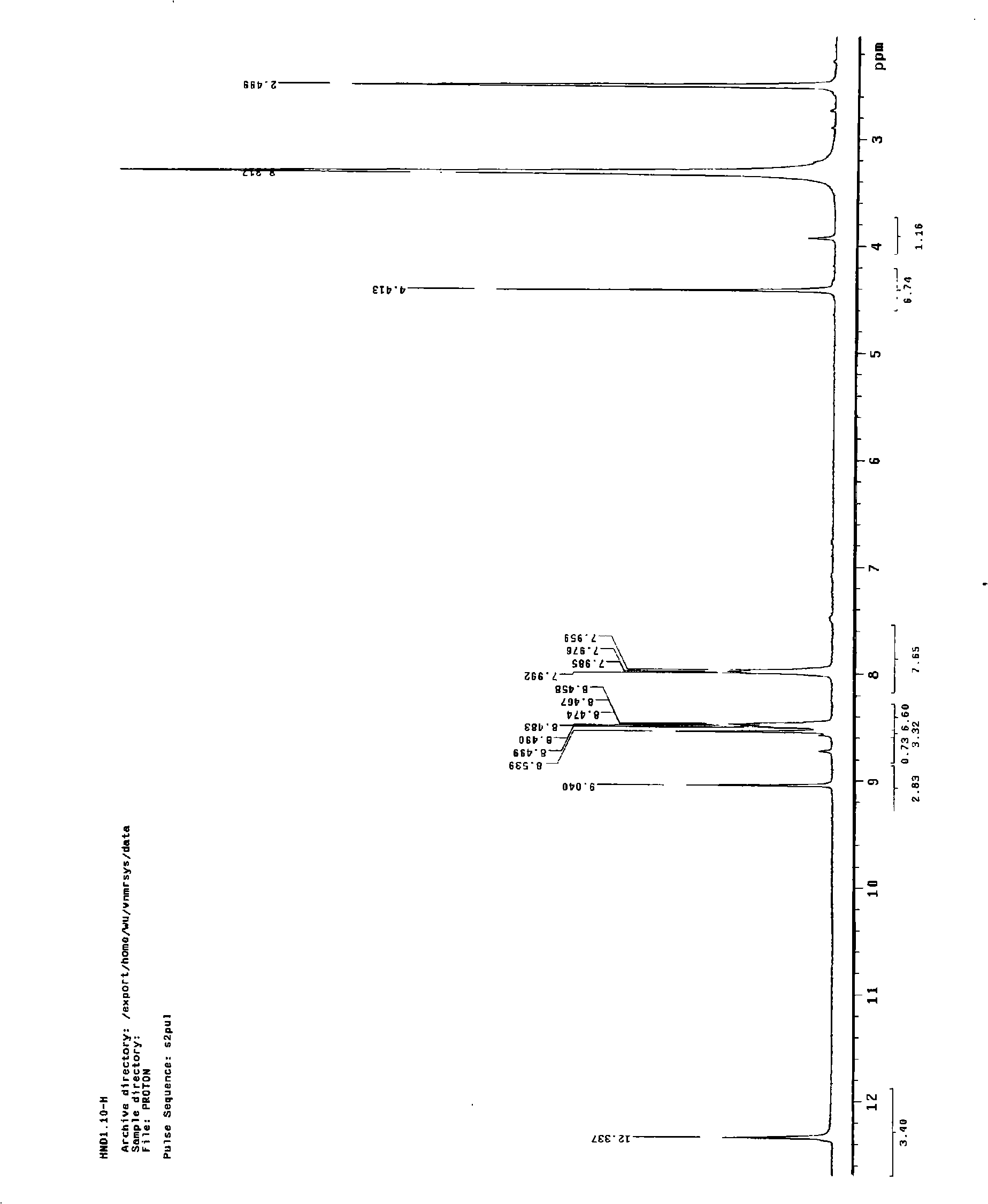
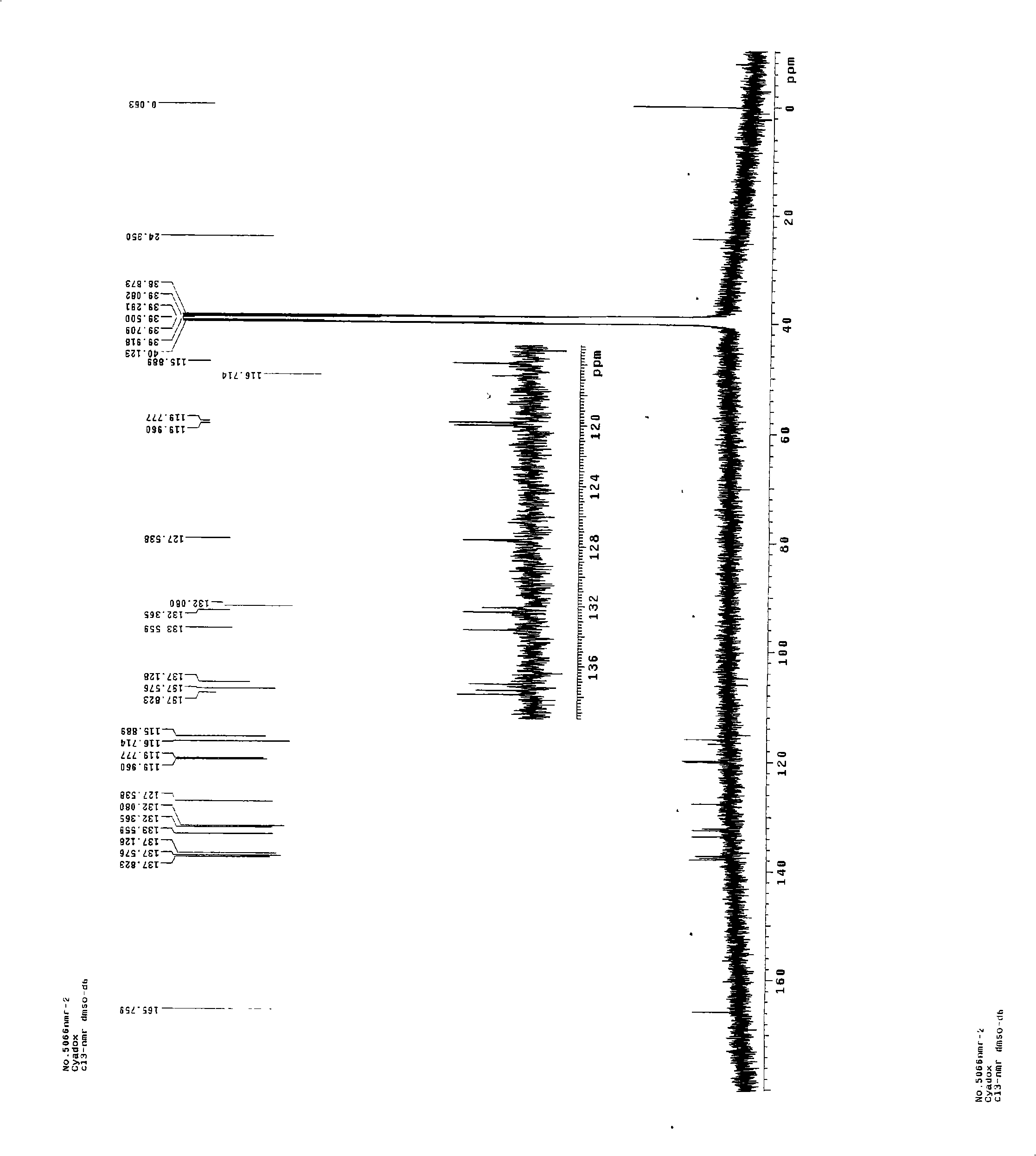
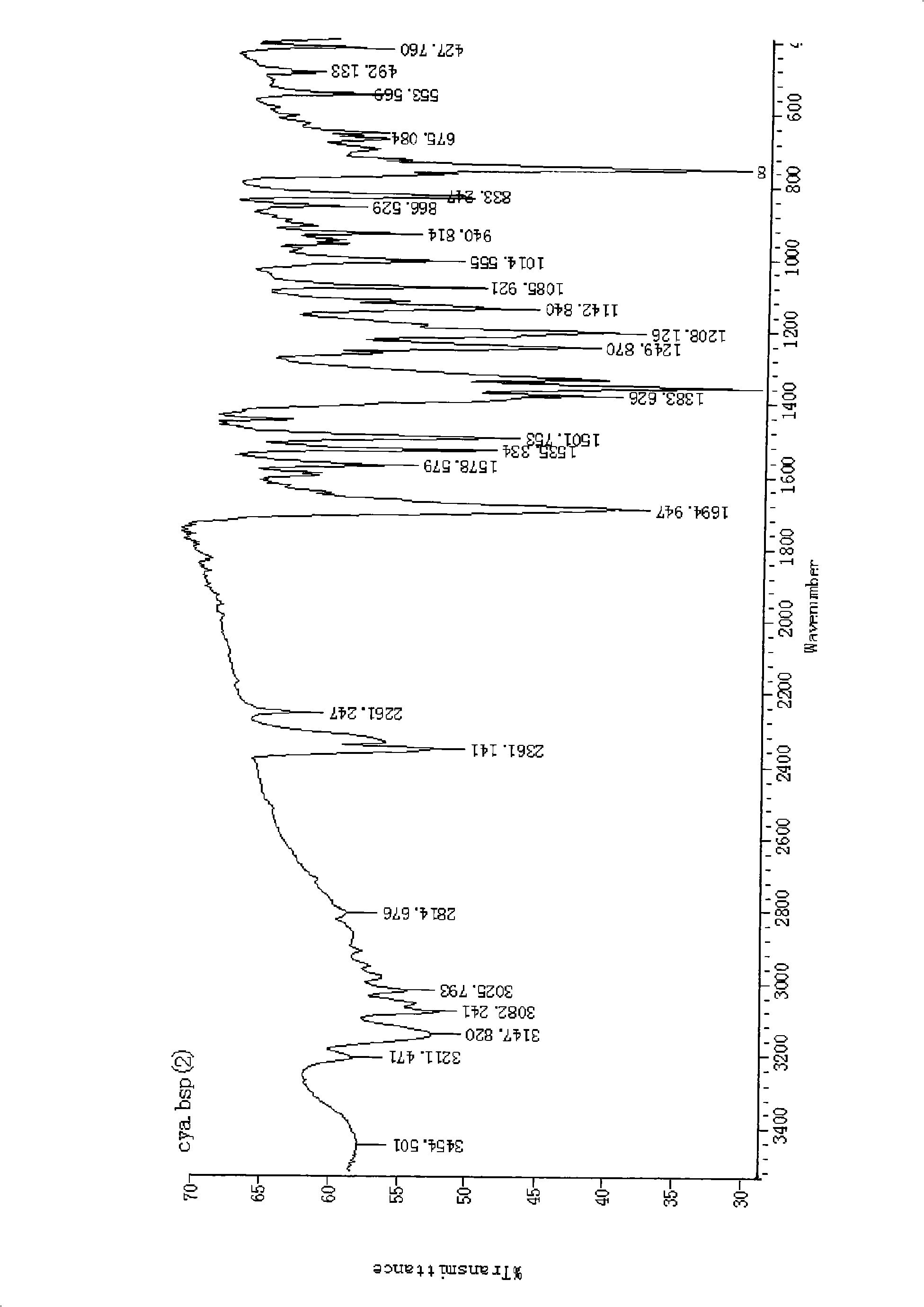
Chemical synthesis method for 2-formylcyanoacetylhydrazone-quinoxaline-1,4-dioxide
A formyl cyanoacetyl hydrazone and dioxide technology, applied in the field of pharmaceutical synthesis, can solve the problems of harsh reaction conditions, difficult reaction control, severe reaction conditions and the like, and achieves short reaction route, low production cost and simple reaction equipment. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Accurately add 27.2 g (0.2 mol) of benzofurazan, 35 mL of N, N-dimethylformamide and aceguvaldehyde dimethyl acetal to a conventional 500 mL four-necked flask equipped with an electric stirrer, a thermometer and a dropping funnel 23.6g, stir to dissolve, when a clear and transparent solution is formed, slowly drop 3mL of pyrrolidine into the reaction with a dropping funnel, the whole reaction system is reacted at 5°C in the dark for 12h, after the reaction is completed, let it stand for 1h, and pump it under reduced pressure filter, wash the filter cake with 50 mL of methanol, and dry it in an oven at 70°C for 2 hours to obtain 23.6 g of quinoxaline-1,4-dioxo-2-carbaldehyde dimethyl acetal as light yellow solid powder, with a yield of 50%.
[0030] Accurately add 5.9 g (0.025 mol) of quinoxaline-1,4-dioxo-2-carbaldehyde dimethyl acetal to a conventional 500 mL four-necked flask equipped with an electric stirrer, a thermometer and a dropping funnel, add 90 mL of methanol ...
Embodiment 2
[0032] Accurately add 27.2 g (0.2 mol) of benzofuroxan, 45 mL of N, N-dimethylformamide and aceguvaldehyde dimethyl acetal to a conventional 500 mL four-necked flask equipped with an electric stirrer, a thermometer and a dropping funnel 47.2g, stir and dissolve, when a clear and transparent solution is formed, slowly drop 5mL of pyrrolidine into the reaction with a dropping funnel, the whole reaction system is reacted at 20°C in the dark for 20h, after the reaction is completed, let it stand for 1h, pump it under reduced pressure Filter, rinse the filter cake with 50 mL of methanol, and dry in an oven at 70° C. for 2 h to obtain 28.9 g of quinoxaline-1,4-dioxo-2-carbaldehyde dimethyl acetal as light yellow solid powder, with a yield of 62%.
[0033] Accurately add 5.9 g (0.025 mol) of quinoxaline-1,4-dioxo-2-carbaldehyde dimethyl acetal to a conventional 500 mL four-necked flask equipped with an electric stirrer, a thermometer and a dropping funnel, add methanol 125 mL and Con...
Embodiment 3
[0035] Accurately add 27.2 g (0.2 mol) of benzofuroxan, 60 mL of N, N-dimethylformamide and aceguvaldehyde dimethyl acetal to a conventional 500 mL four-neck flask equipped with an electric stirrer, a thermometer and a dropping funnel 82.6g, stirring and dissolving, when a clear and transparent solution is formed, slowly drop 5mL of pyrrolidine into the reaction with a dropping funnel, and the whole reaction system is reacted at 30°C in the dark for 24h. Filter, rinse the filter cake with 50 mL of methanol, and dry in an oven at 70°C for 2 hours to obtain 26.8 g of quinoxaline-1,4-dioxo-2-carbaldehyde dimethylacetal as a light yellow solid powder, with a yield of 57%.
[0036] Accurately add 5.9 g (0.025 mol) of quinoxaline-1,4-dioxo-2-carbaldehyde dimethyl acetal to a conventional 500 mL four-necked flask equipped with an electric stirrer, a thermometer and a dropping funnel, add 150 mL of methanol and Concentrated hydrochloric acid 110mL, carry out alcoholysis under the condit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com