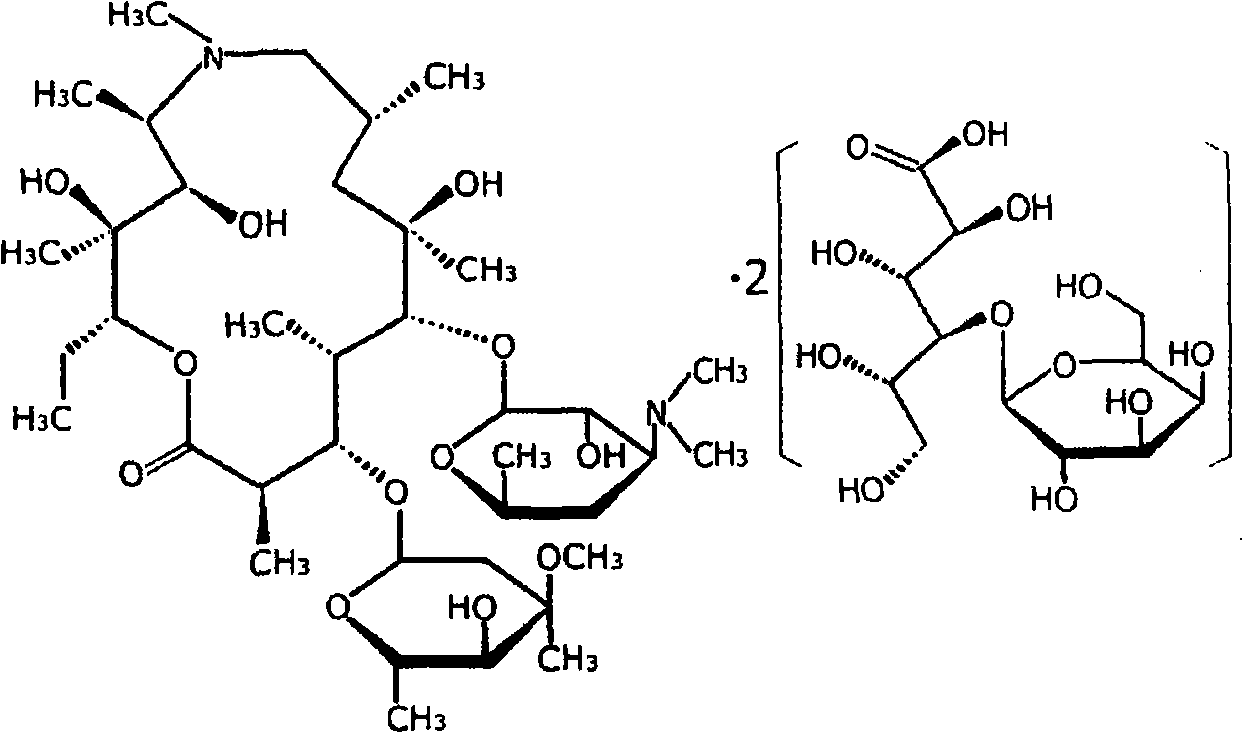
Lactobionic acid azithromycin for injections and preparation method thereof
A technology of azithromycin and lactobionic acid, applied in the field of azithromycin lactobionate freeze-dried powder injection and its preparation, can solve the problems of reduced potency, increased toxicity, loss of antibacterial activity, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
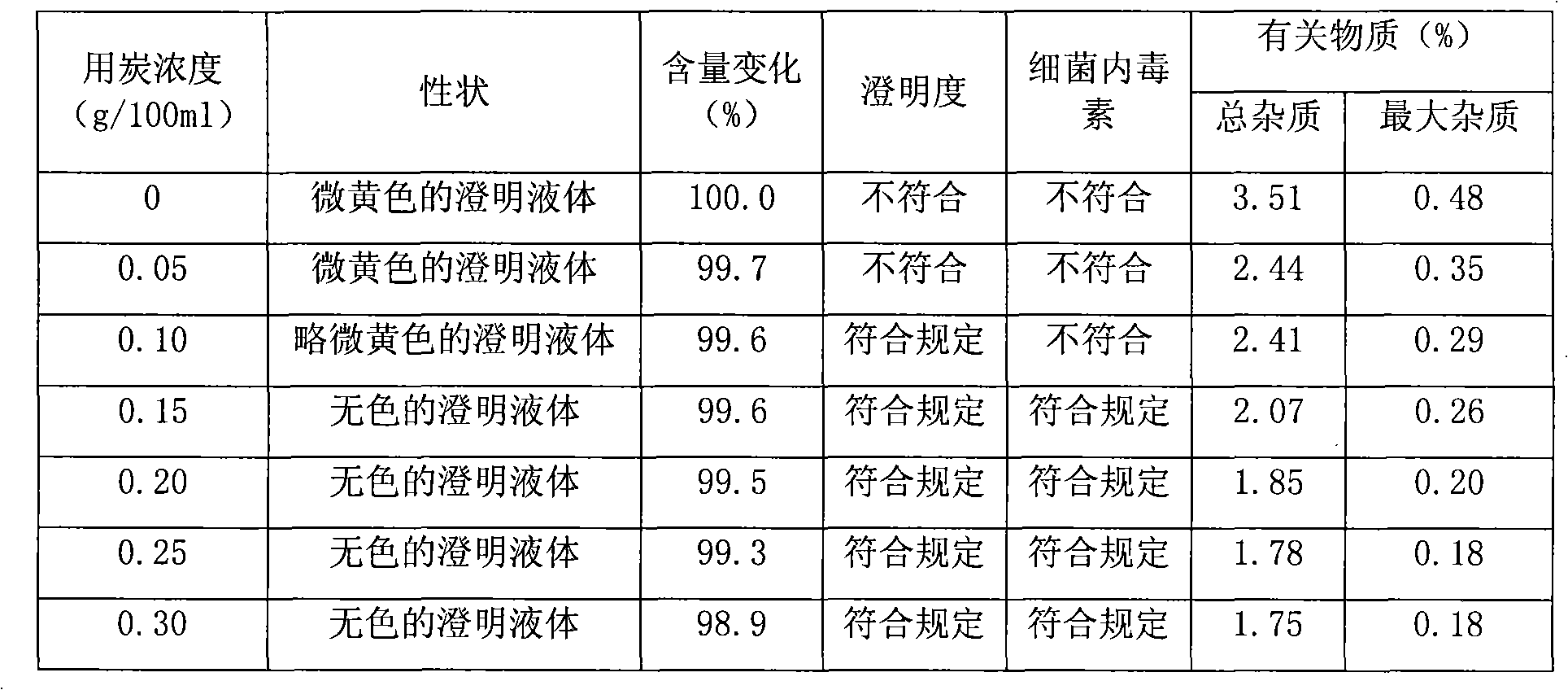
[0035] Dissolve 250g of azithromycin lactobionate finished product in 1600mL of water for injection, stir to dissolve, add 4.0g of activated carbon, stir and decolorize at a temperature of 5°C for 30 minutes, then filter through a 0.45μm microporous membrane to remove carbon, and then add water for injection to 2000ml, fully stirred for 20 minutes to mix the solution evenly, and the prepared solution was filtered and sterilized through a 0.22 μm microporous membrane. Put the final filtrate into the tray of the lyophilizer, quickly cool down to -38°C to make it initially solidify, then continue to cool down to -55°C, keep it frozen at -55°C for 4 hours, make it completely frozen, vacuumize, and then Gradually raise the temperature to -2°C within 11 hours, maintain -2°C and vacuum dry for 8 hours, continue to raise the temperature, rise to 30°C within 3 hours, and maintain 30°C for 7 hours to obtain azithromycin lactobionate freeze-dried powder injection The moisture in it is 0....
Embodiment 2
[0037] Dissolve 250g of the finished product of azithromycin lactobionate in 1600mL of water for injection, stir to dissolve, add 3.2g of activated carbon, stir and decolorize at a temperature of 3°C for 30 minutes, then filter through a 0.45μm microporous membrane to remove carbon, and then add water for injection to 2000ml, fully stirred for 20 minutes to mix the solution evenly, and the prepared solution was filtered and sterilized through a 0.22 μm microporous membrane. Put the final filtrate into the tray of the lyophilizer, quickly cool down to -35°C to make it initially solidify, then continue to cool down to -60°C, keep it frozen at -60°C for 4.5 hours, make it completely frozen, vacuumize, and then Gradually raise the temperature to 0°C within 12 hours, maintain 0°C and vacuum-dry for 9 hours, continue to raise the temperature, rise to 35°C within 3.5 hours, and maintain 35°C for 6.5 hours, the obtained azithromycin lactobionate freeze-dried powder injection Moisture ...
Embodiment 3
[0039] Dissolve 250g of the finished product of azithromycin lactobionate in 1600mL of water for injection, stir to dissolve, add 3.68g of activated carbon, stir and decolorize at a temperature of 8°C for 30 minutes, then filter through a 0.45μm microporous membrane to remove carbon, and then add water for injection to 2000ml, fully stirred for 20 minutes to mix the solution evenly, and the prepared solution was filtered and sterilized through a 0.22 μm microporous membrane. Put the final filtrate into the feed tray of the freeze dryer, quickly cool down to -30°C to make it initially solidify, then continue to cool down to -50°C, keep it frozen at -50°C for 5 hours, make it completely frozen, vacuumize, and then Gradually raise the temperature to -5°C within 9 hours, maintain -5°C for 11 hours in vacuum drying, continue to raise the temperature, rise to 30°C within 2 hours, maintain 30°C for 6 hours in vacuum drying, and obtain azithromycin lactobionate freeze-dried powder inje...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com