Wet chemical preparation method containing spherical silver powder
A technology of wet chemistry and silver powder, which is applied in the field of preparation of ultra-fine dense spherical silver powder, can solve the problems of many steps, toxicity, and strict conditions, and achieve the effects of good dispersion, good sphericity, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
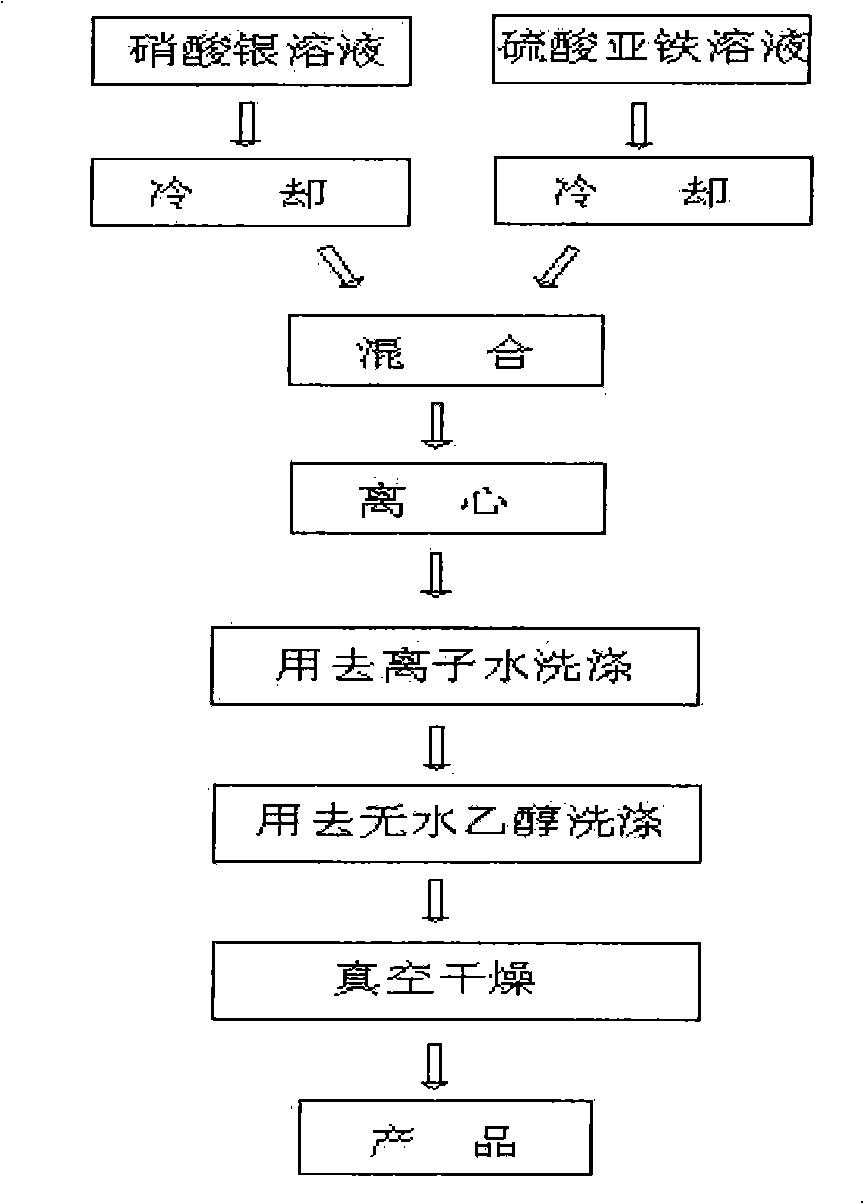
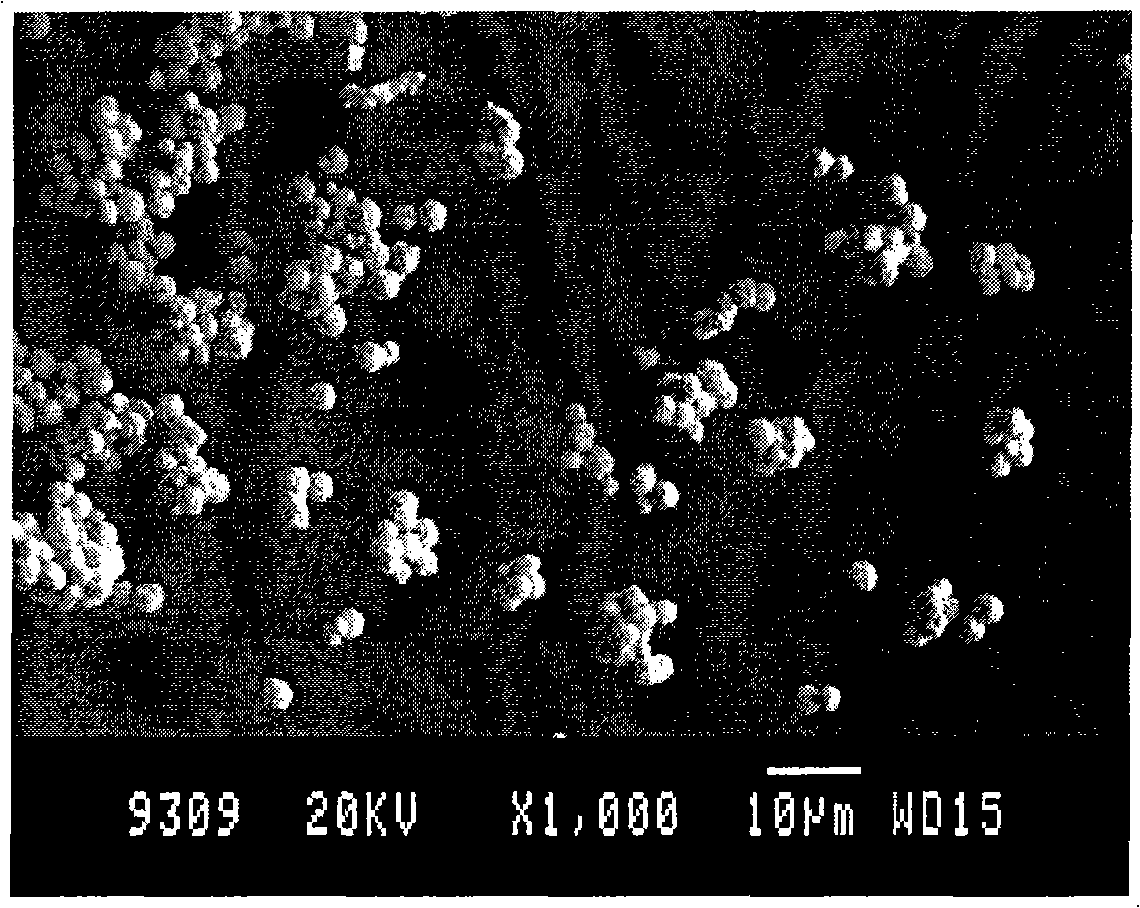
[0019] Embodiment one: press figure 1 The process flow shown is implemented. Weigh 1g of silver nitrate and dissolve it in deionized water to 50ml, weigh 4.4g of FeSO 4 ·7H 2 O was dissolved in deionized water to a volume of 50ml. Both were cooled to 6°C. Then mix the two completely and homogeneously as soon as possible under stirring conditions. until the color of the solution does not change. Centrifuge, wash with deionized water 3 times, then wash 2 times with absolute ethanol, and vacuum dry at 50°C to obtain silver powder. The obtained silver powder is as figure 2 As shown, it has good sphericity, average particle size of 3.0 μm, good dispersion, narrow particle size distribution and high purity.
Embodiment 2
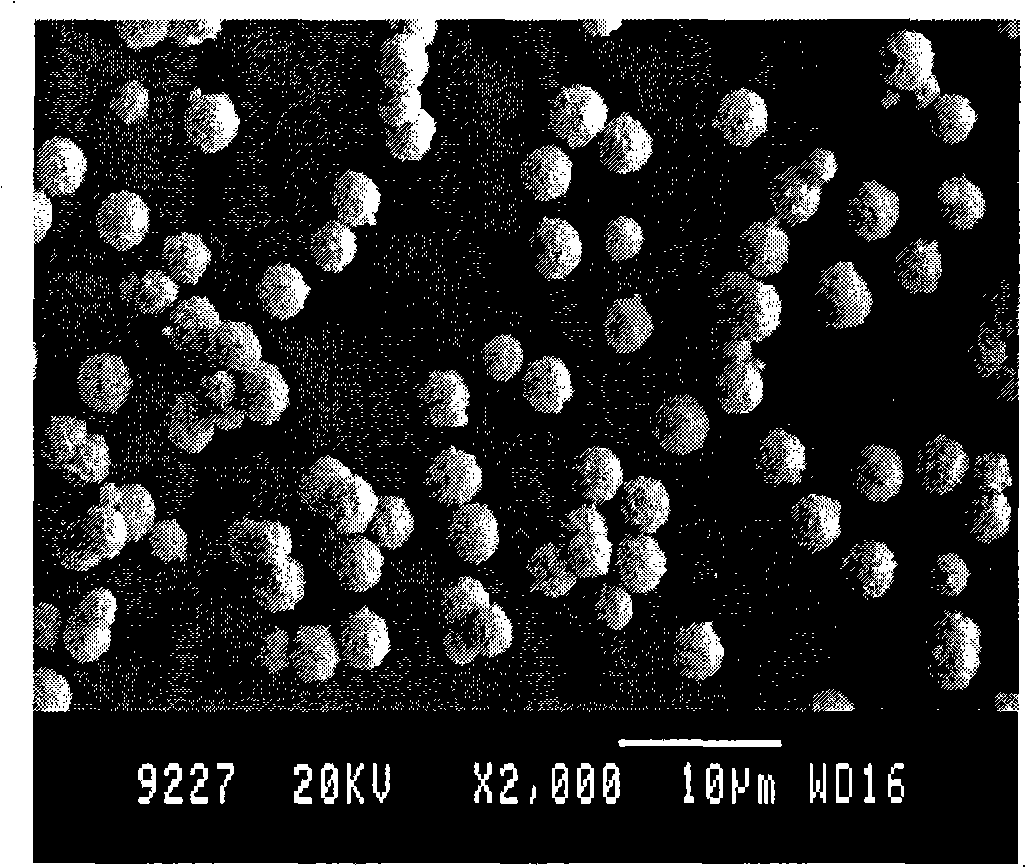
[0020] Embodiment 2: Take by weighing 2g of silver nitrate and dissolve it in deionized water to settle to 50ml, weigh 8.8g of FeSO 4 ·7H 2 O was dissolved in deionized water to a volume of 50ml. Both were cooled to 6°C. Then mix the two completely and homogeneously as soon as possible under stirring conditions. until the color of the solution does not change. The resulting silver powder was precipitated by centrifugation, washed three times with deionized water, then washed twice with absolute ethanol, and vacuum-dried at 50° C. to obtain silver powder. The obtained silver powder is as image 3 As shown, it has good sphericity, average particle size of 3.5 μm, good dispersion, narrow particle size distribution and high purity. The energy spectrum component analysis of gained silver powder is as follows: Image 6 , the particle size distribution of which is Figure 7 .
Embodiment 3
[0021] Embodiment three: take by weighing 1.2g silver nitrate and dissolve and settle to 50ml with deionized water, take by weighing 8.8gFeSO 4 ·7H 2 O was dissolved in deionized water to a volume of 50ml. Both were cooled to 20°C. Then mix the two completely and homogeneously as soon as possible under stirring conditions. until the color of the solution does not change. The precipitated silver powder was separated by centrifugation, washed three times with deionized water, then washed three times with absolute ethanol, and dried in vacuum at 50°C to obtain silver powder. The obtained silver powder is as Figure 4 As shown, it has good sphericity, average particle size of 1.7 μm, good dispersion, narrow particle size distribution and high purity.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com