Method for synthesizing albendazole
A kind of technology of albendazole and synthetic method, applied in the field of organic synthesis, can solve the problems of high cost, large amount of glacial acetic acid, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
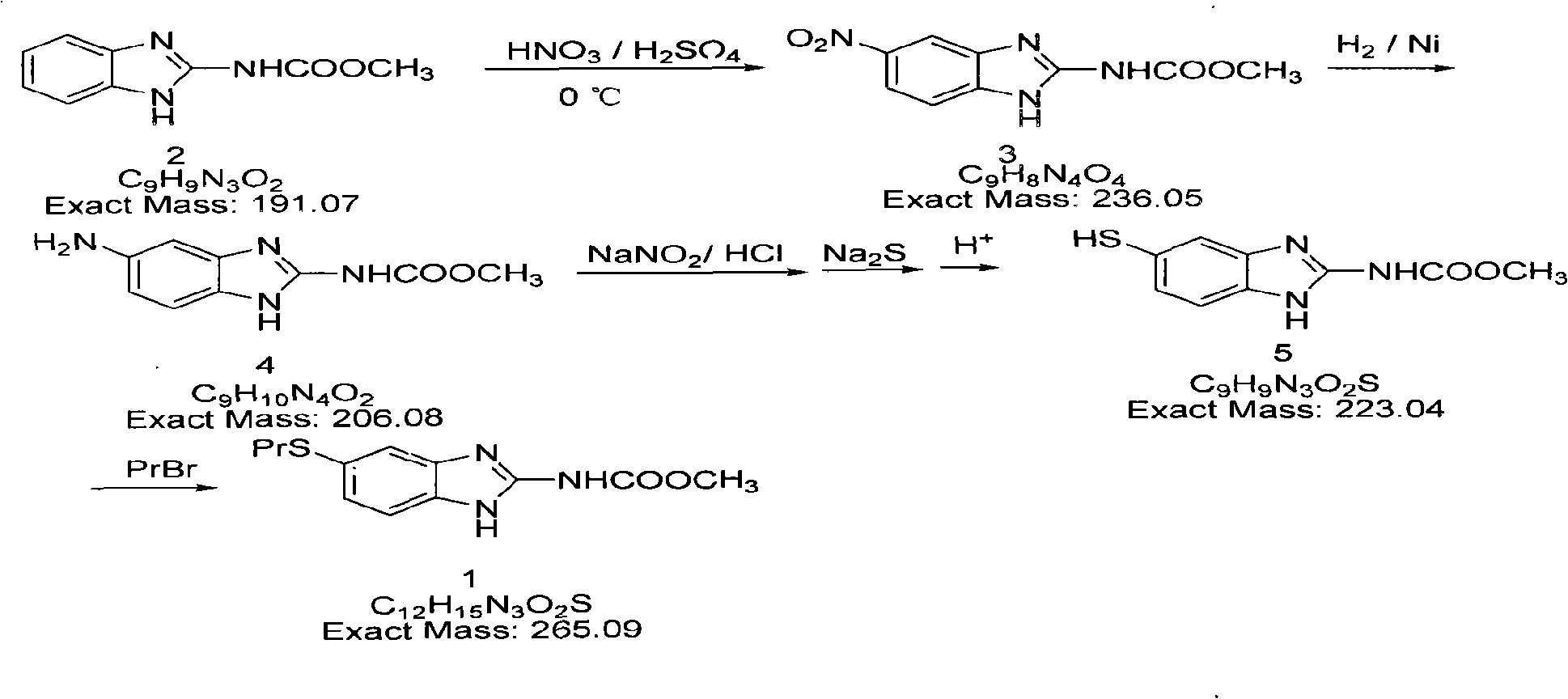
[0013] The preparation of 5-nitrobenzimidazole-2-carbamate methyl ester (3): in 500ml there-necked flask, add benzimidazole-2-carbamate methyl ester 20g (0.1mol), concentrated sulfuric acid 20ml, reduce to 0 ℃, and controlled below 0~5℃, add 20ml of fuming nitric acid dropwise, after the dropwise addition, keep warm and stir for reaction (below 10℃) for 2h. The reactant was poured into ice water, and a light yellow solid was precipitated, which was suction filtered, washed with water, and dried to obtain (3) 18.2 g of a light yellow solid, yield 77.0%, mp: 112-114°C.
[0014] The preparation of 5-aminobenzimidazole-2-methyl carbamate (4): add 17g (3) (0.07mol) and 2g of bone nickel suspended in 30ml absolute ethanol to a 250ml autoclave, turn off the high pressure The kettle, add hydrogen from the steel cylinder (pressure 7.5MPa, temperature 6°C), start the reaction at about 55°C, and keep the pressure in the kettle at 0.05-0.30MPa. When the hydrogen is no longer absorbed (ab...
Embodiment 2
[0020] The preparation of 5-nitrobenzimidazole-2-methyl carbamate (3): in a 500ml there-necked flask, add 22g (0.11mol) of benzimidazole-2-methyl carbamate, 30ml of concentrated sulfuric acid, and reduce to 0 Below 5°C, and controlled below 5°C, add 20ml of fuming nitric acid dropwise, after the dropwise addition, stir the reaction (below 5°C) for 2 hours, pour the reactant into ice water, and precipitate a pale yellow solid, filter with suction, wash with water, and dry to obtain ( 3) 20.3 g of light yellow solid, yield 78.0%, mp: 112-114°C.
[0021] Preparation of methyl 5-aminobenzimidazole-2-carbamate (4): Add 25.5 g (3) (0.105 mol) and 3 g of bone nickel suspended in 50 ml of absolute ethanol to a 250 ml autoclave, close In the autoclave, add hydrogen from the steel cylinder (pressure 75atm, temperature 6°C), start the reaction at about 60°C, and keep the pressure in the kettle at 0.05-0.30Mpa. When the hydrogen is no longer absorbed (about 60 ~ 90), turn off the heater,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com