Method for preparing 7 alpha-bromo-sterides
A steroidal compound, brominated technology, applied in the field of preparation of 7α-bromosteroidal compound, can solve the problems of improvement and large amount of use, and achieve the effects of simple operation, low production cost, and environmentally friendly process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
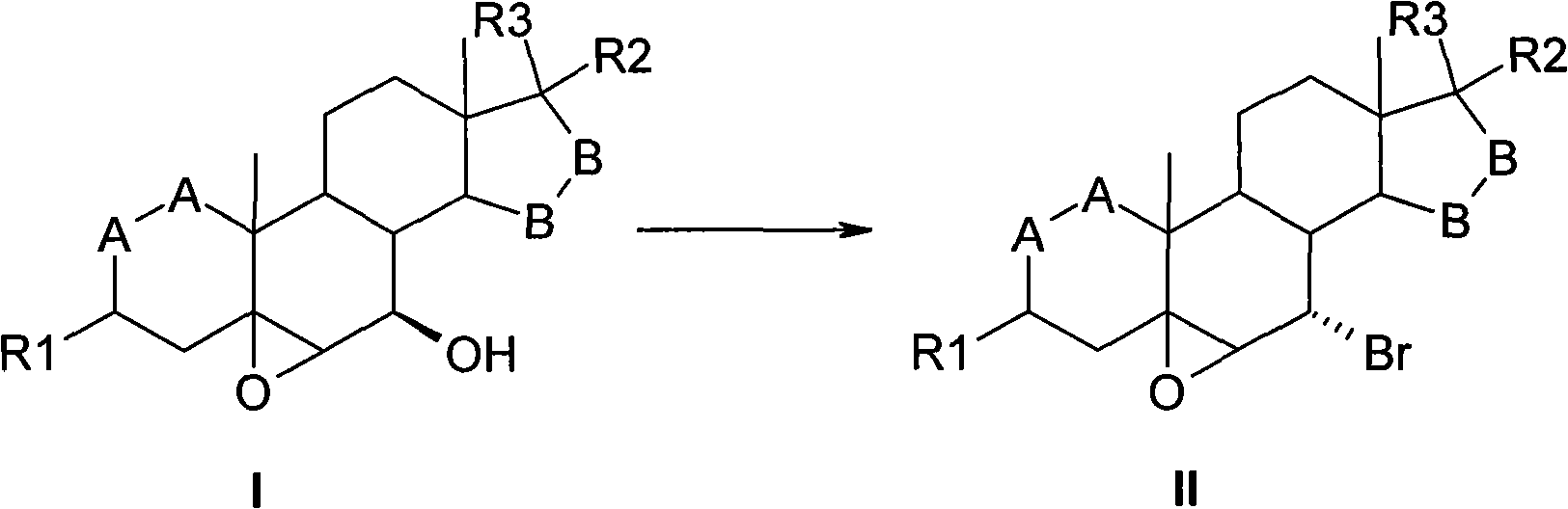
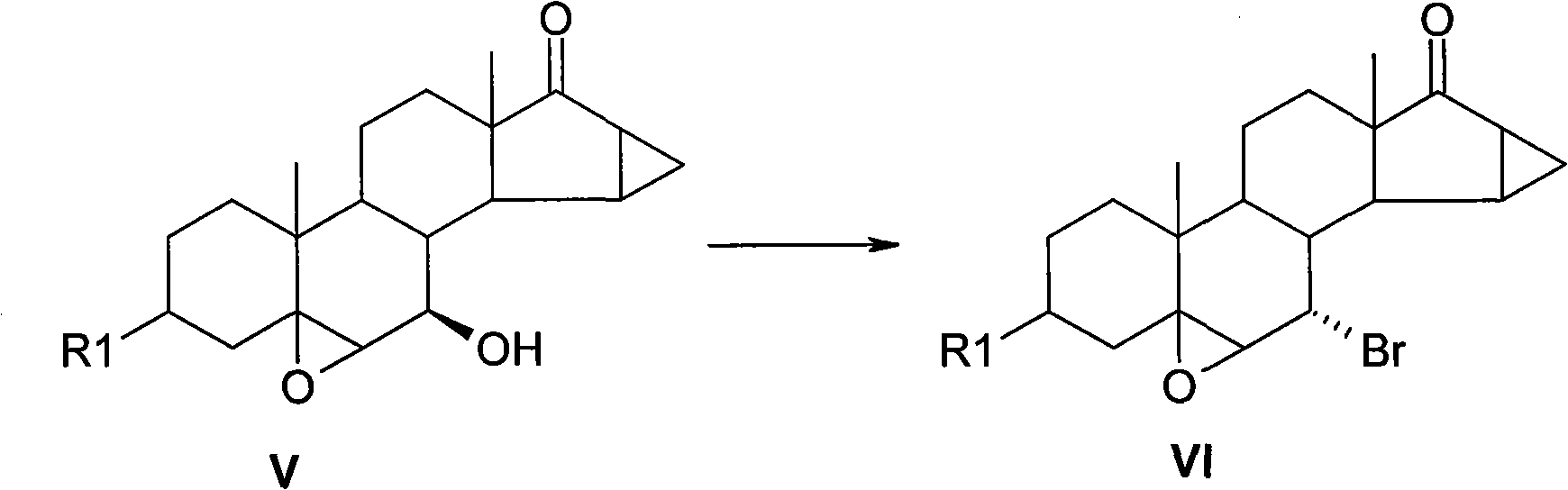
[0034] Under the protection of argon, put 10g of 3β-pivaloyloxy-5,6β-epoxy-7β-hydroxyl-15β,16β-methylene-androst-17-one into the reaction flask, add dichloromethane 20ml, and 20ml pyridine, keep the temperature at 25°C, add 11.8g triphenylphosphine after it dissolves, dilute 1.3ml liquid bromine with 5ml dichloromethane, drop it into the reaction flask within 1 hour, and drop it off The reaction was then stirred for about 2 hours.
[0035] The reaction was followed by TLC (developing solvent: cyclohexane: ethyl acetate = 1:4).
[0036] After the reaction was completed, water was added to terminate the reaction, the organic phase was extracted and separated, and the organic phase was washed twice with water. All aqueous phases were combined and back extracted with dichloromethane. All organic phases were combined and dried over anhydrous sodium sulfate for 30 minutes. Concentrate to dryness, add methanol twice, and concentrate again to take away residual dichloromethane. Me...
Embodiment 2
[0038] Under the protection of argon, put 10g of 3β-pivaloyloxy-5,6β-epoxy-7β-hydroxyl-15β,16β-methylene-androst-17-one into the reaction flask, add dichloromethane 20ml, and 20ml pyridine, keep the temperature at 25°C, add 8.2g triphenylphosphine after the solution is clear, dilute 1.5ml liquid bromine with 5ml dichloromethane, drop it into the reaction flask within 1 hour, drop it off The reaction was then stirred for about 4 hours.
[0039] Post-reaction treatment is the same as in Example 1.
[0040] The product 3β-pivaloyloxy-5,6β-epoxy-7α-bromo-15β,16β-methylene-androst-17-one was dried to obtain 9.6g, with a yield of 84%, and a purity of 92% by HPLC. %.
Embodiment 3
[0042] Under the protection of argon, put 10g of 3β-pivaloyloxy-5,6β-epoxy-7β-hydroxyl-15β,16β-methylene-androst-17-one into the reaction flask, add dichloromethane 25ml, and 25ml of pyridine, keep the temperature at 20°C, add 14.5g of triphenylphosphine after the solution is clear, dilute 2.0ml of liquid bromine with 6ml of dichloromethane, drop it into the reaction flask within 1 hour, and drop it off The reaction was then stirred for about 2 hours.
[0043] Post-reaction treatment is the same as in Example 1.
[0044] The product 3β-pivaloyloxy-5,6β-epoxy-7α-bromo-15β, 16β-methylene-androst-17-one 8.1g was obtained by drying, the yield was 70%, and the purity by HPLC was 90%. %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com