Preparation of a crystalline antibiotic substance
A technology of fusidic acid and crystallization, which is applied in the field of preparation of crystalline antibiotic fusidic acid and can solve problems such as affecting the quality of medicines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0076] In a more particular aspect, the present invention relates to a mixture of crystalline forms of fusidic acid comprising crystalline fusidic acid of the invention as described above, wherein the mixture of crystalline forms of fusidic acid consists essentially of crystalline fusidic acid half Hydrate composition.
[0077] In another more particular aspect, the present invention relates to a mixture of crystalline forms of fusidic acid comprising crystalline fusidic acid according to the invention as described above, wherein the mixture of crystalline forms of fusidic acid comprising is characterized in that it exhibits Crystalline fusidic acid characterized by one or both of t) or u):
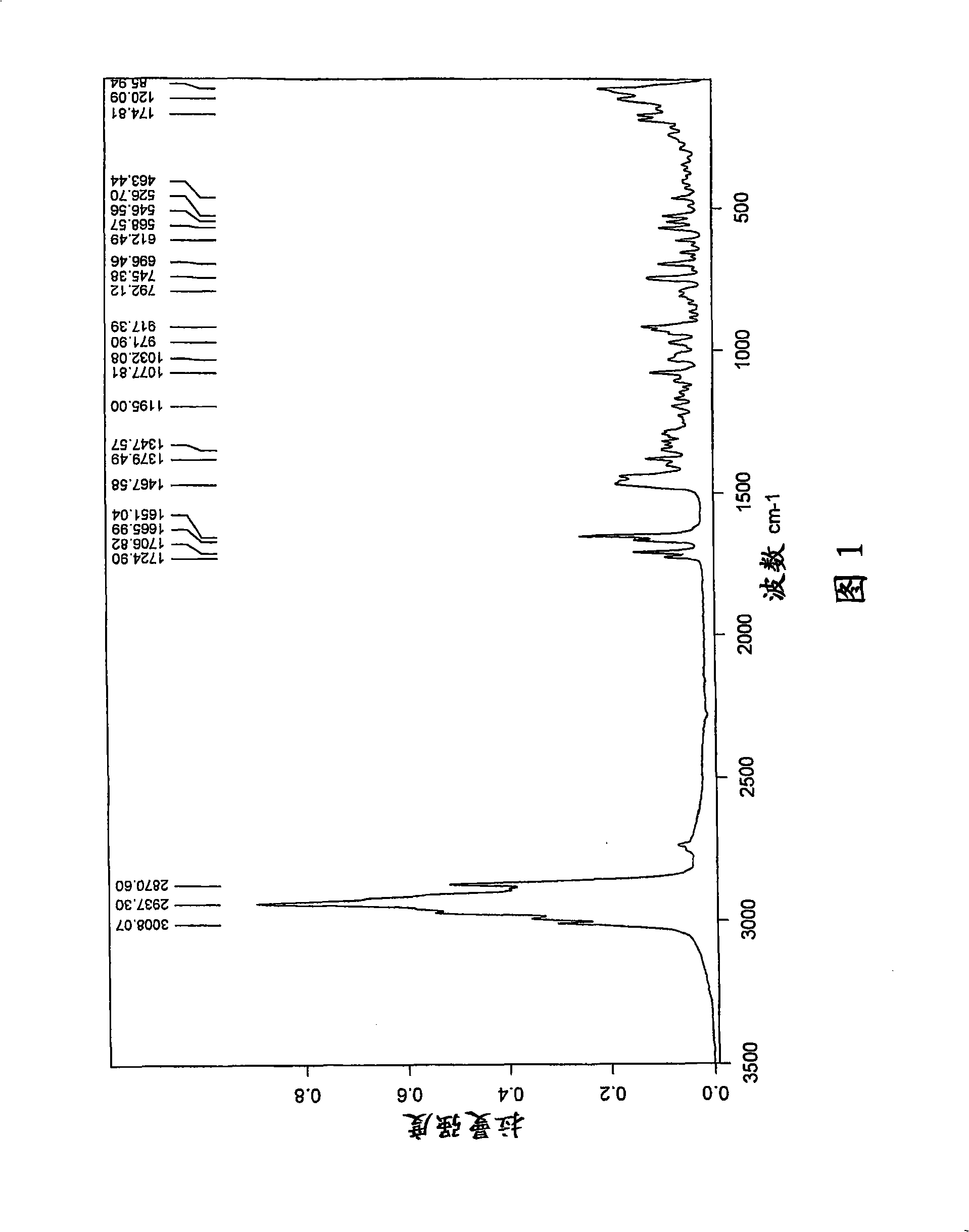
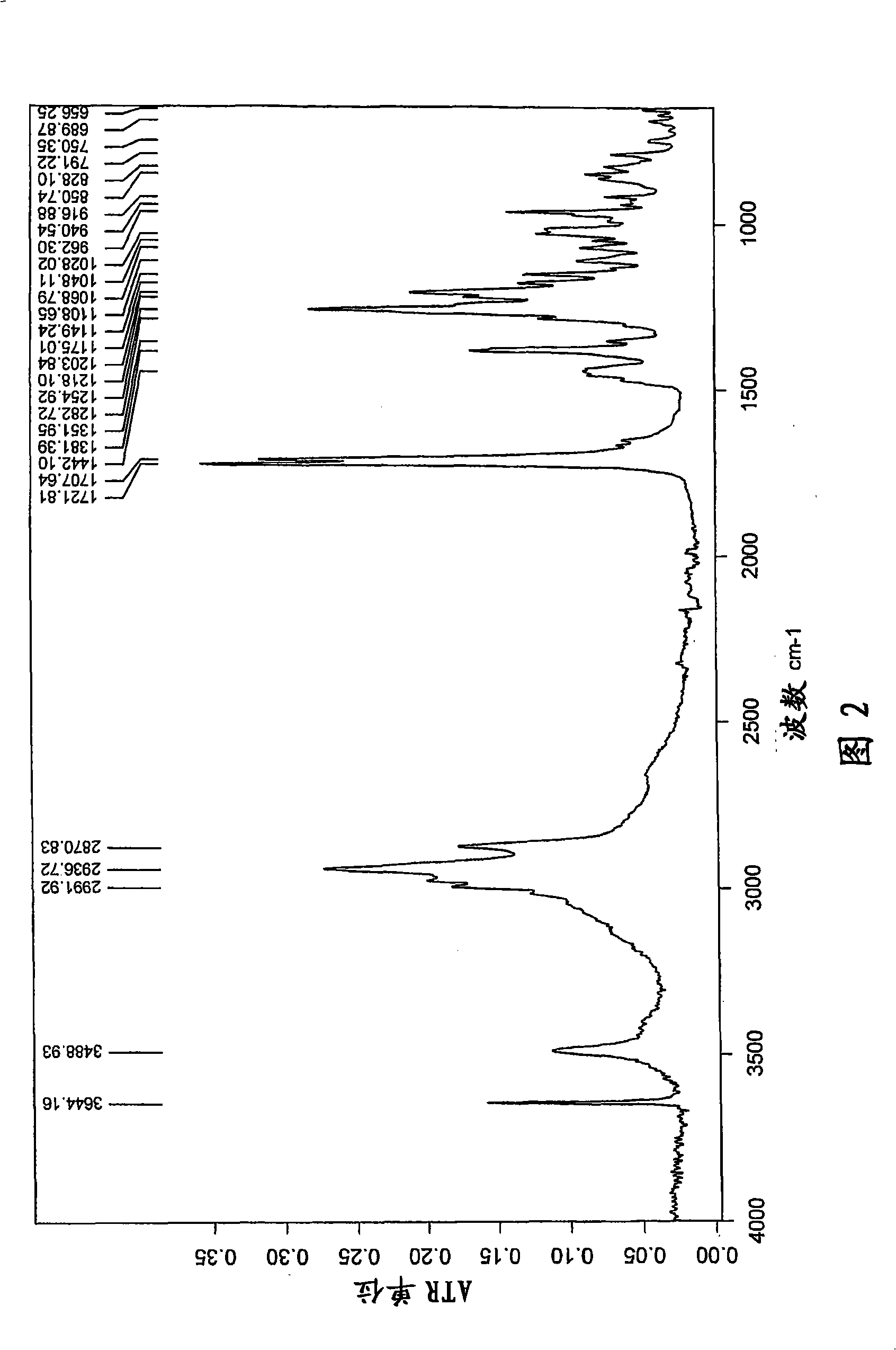
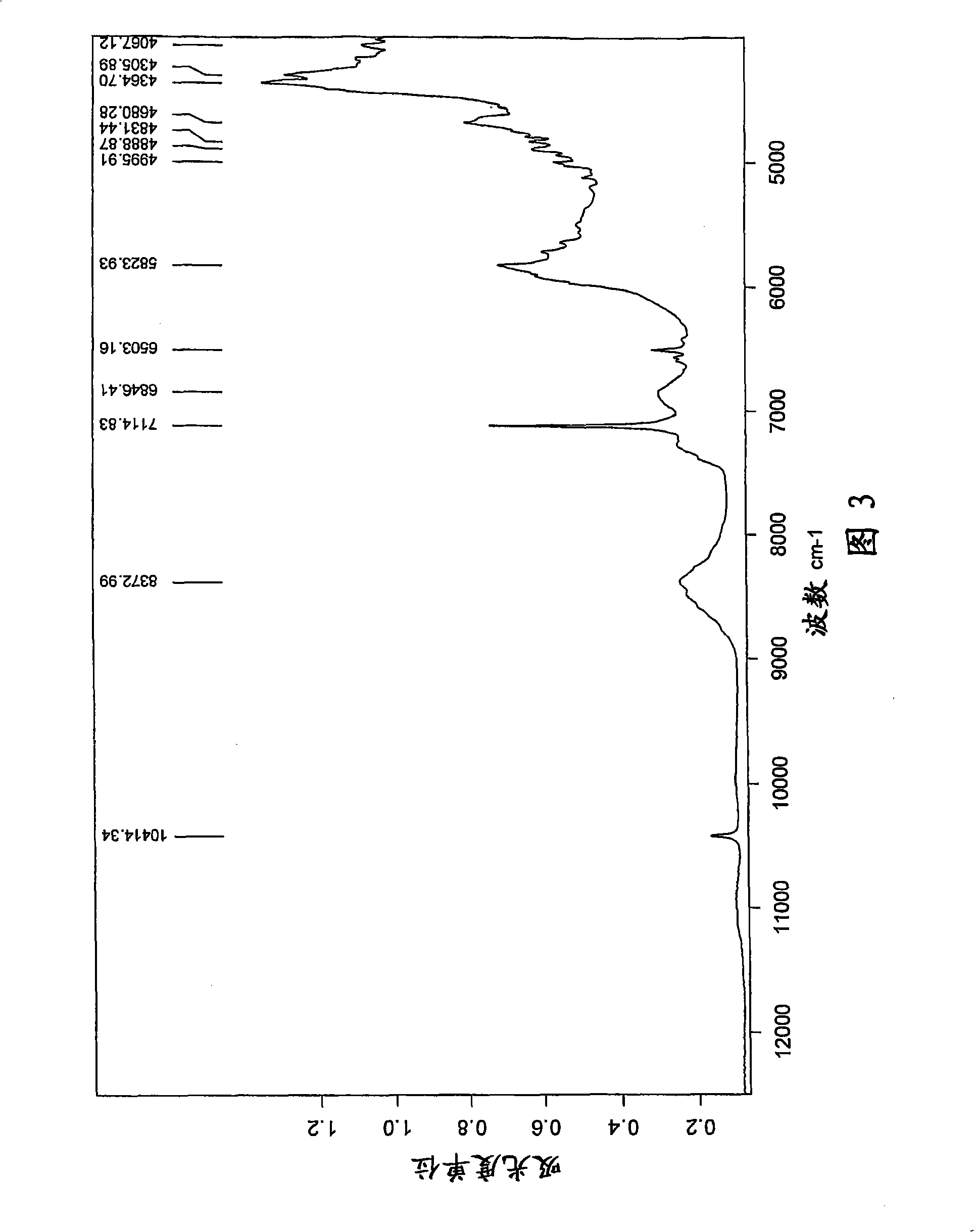
[0078] t) Infrared (FT-IR) spectrum (KBr) with one or more -1 ); or
[0079] u) X-ray powder diffraction pattern (XRD) having one or more 2θ values (±0.1) occurring at about 2.1, 6.8, 9.4, 10.4, 11.8, 12.8, 13.7, 14.2, 15.8, 17.3, 18.5, or 22.9, respectively A characteristic intensit...
Embodiment 1
[0406] Fusidic acid hemihydrate
[0407] 17.68 kg of fusidic acid starting material obtained by fermentation was dissolved in a mixture of 69.3 L of ethanol (96%) and 2.52 L of acetone, thereby obtaining 85.5 L of the first solution. Add the first solution and 93.4 L horizontally to a vessel at ambient temperature over 17-19 minutes while mixing. Crystallization was observed immediately after mixing the solution with anti-solvent water. The mixture was mixed further and crystalline fusidic acid hemihydrate was filtered off, washed with a mixture of water and ethanol (3:1, v:v) and water. A micronized sample of this crystal (jet mill) had an endothermic peak at 186°C in DSC (20°C / min), with an onset temperature of 183°C. The crystals are vacuum dried at 50° C. for about 15-18 hours to obtain a crystalline hemihydrate characterized by exhibiting one or more of the following characteristics l)-s), respectively yes:
[0408] l) Fourier transform (FT-NIR) Raman spectrum has one...
Embodiment 2
[0417] Fusidic acid hemihydrate
[0418] 1.64 g of fusidic acid, 313 ml of ethanol, 380 ml of water and 162 mg of acetone were mixed and the saturated solution was filtered through a filter. 178 g of fusidic acid was dissolved in 693 ml of ethanol and 25 ml of acetone and the solution was filtered with a filter. The first solution was poured into a 5 liter flask and stirred in a water bath at 180 rpm at a temperature of 30°C. This solution was seeded with 0.45 g of fusidic acid hemihydrate having the properties described in Example 1. The second solution and water (934ml) were added to the flask in parallel at a rate of 7-8ml / min for a total addition time of 90 minutes. After complete addition, the crystal suspension was stirred for a further 30 minutes. The crystals were filtered off, and the crystals were dried under vacuum at 30°C for 18 hours to obtain crystalline fusidic acid hemihydrate as described in Example 1, which exhibited one or more of the following characteri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com