Therapeutic drug for traumatic neural disease (disorder) and/or motor function disorder
A technology for the treatment of motor dysfunction and drugs, which is applied in the field of drugs for the treatment of traumatic neurological disorders and/or motor dysfunction, and can solve problems such as unclear effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
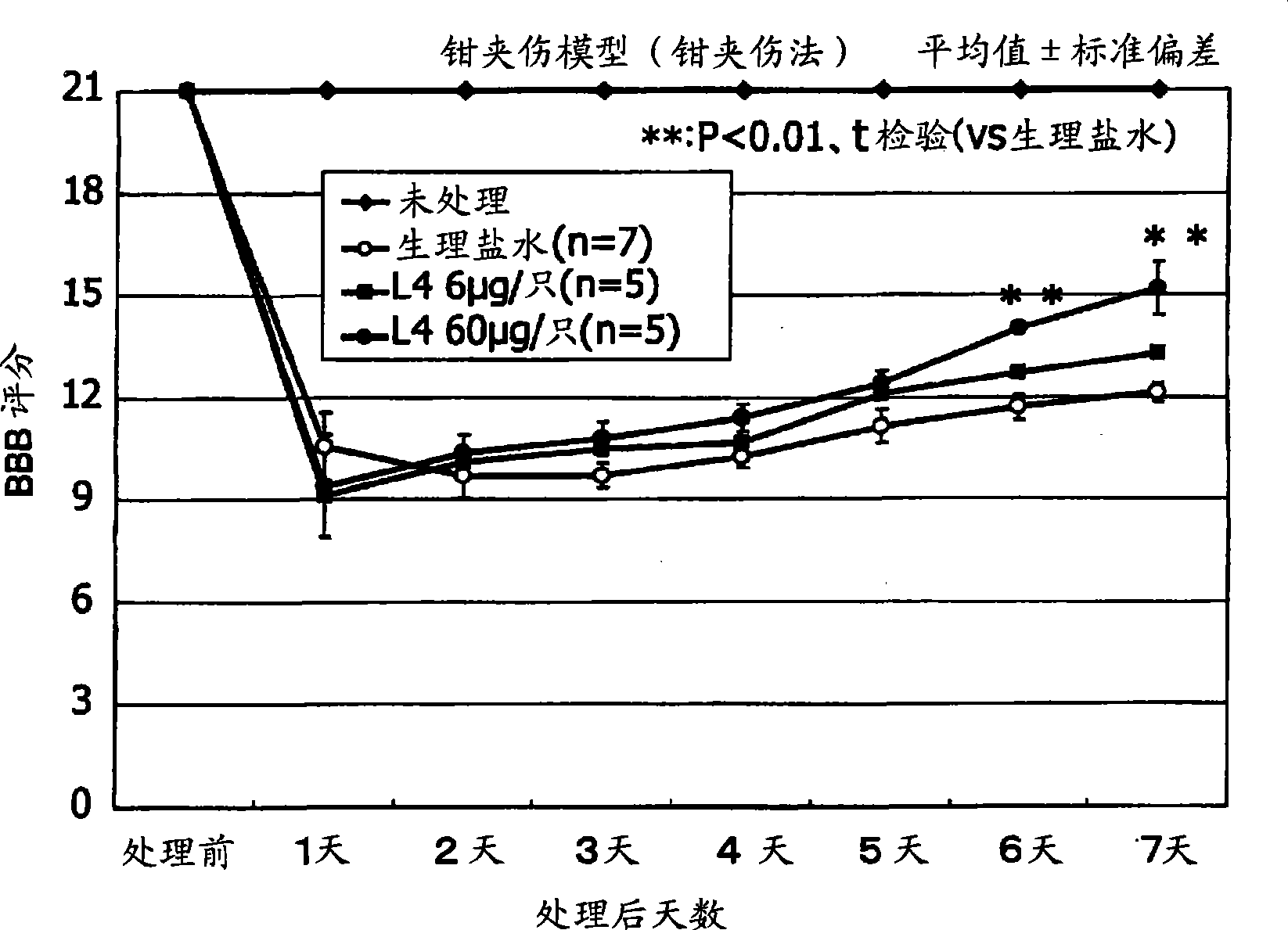
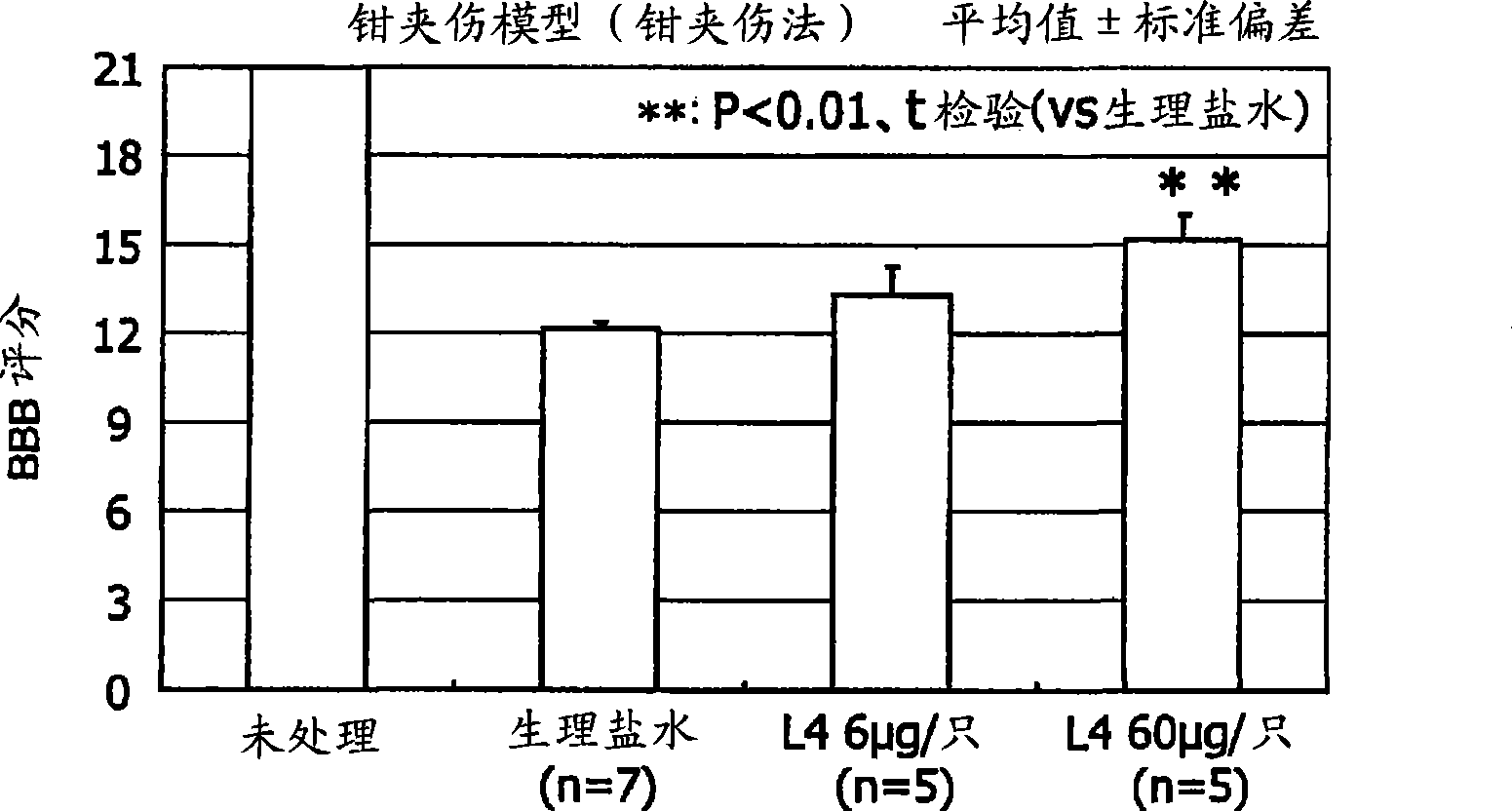
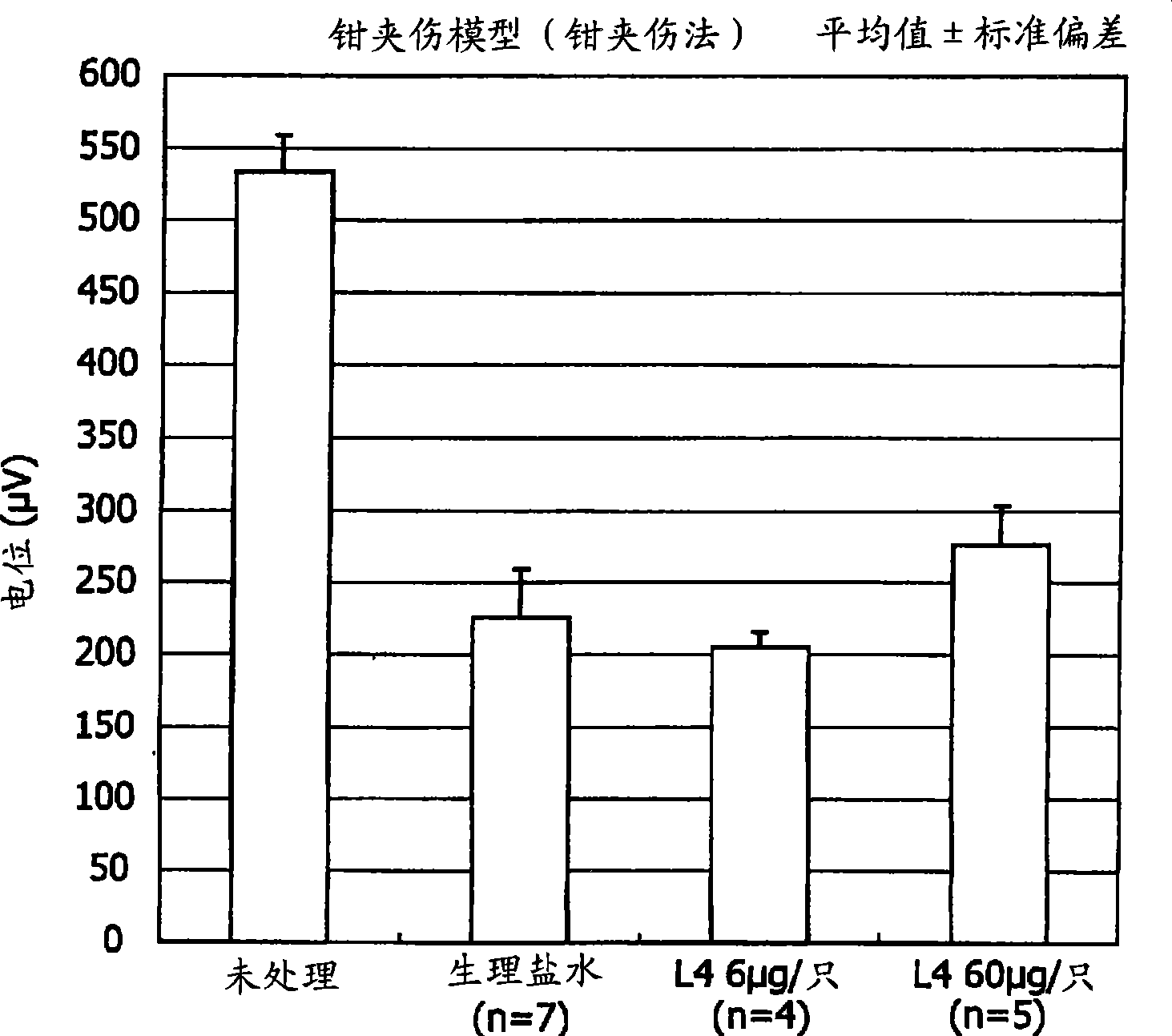
[0072] Embodiments of the present invention will be explained below with reference to the drawings. However, the present invention is not limited to the Examples set forth below.
[0073] [Materials and methods]
[0074] [Preparation of L4]
[0075] Using the method of Xu et al., as described below, L4 was prepared by degradation of keratan sulfate with cutinase II followed by separation by anion exchange chromatography.
[0076] KS oligosaccharide (L4) was isolated from the degradation product of cutanase II from shark fin keratan sulfate (Biochemical Industry) by performing a series of steps of anion exchange chromatography and gel permeation chromatography. The oligosaccharide was identified by capillary electrophoresis and mass spectrometry (Non-Patent Document 4).
[0077] Capillary electrophoresis was performed using a Quanta 4000 capillary electrophoresis system (Waters) with UV detector. In capillary electrophoresis systems, the sample is loaded on the anode, using...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com