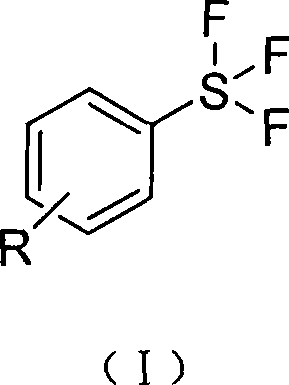
Aryl sulfur fluoride type fluorination reagent and preparation method thereof
A technology of aryl sulfur fluoride and radical sulfur fluoride, which is applied in the field of aryl sulfur fluoride type fluorinated reagents and its preparation, and can solve problems such as instability, low thermal decomposition temperature, and easy explosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Preparation of 4-formic acid thiobenzene trifluoride (I-1a) and 4-ethoxycarbonyl thiobenzene trifluoride (I-1b)
[0049]
[0050] 1. Preparation of methyl 4-(dimethylaminothioformyloxy)benzoate (5)
[0051] Add 30g (0.197mol) methyl p-hydroxybenzoate to a 500mL single-necked bottle, dissolve it with 210mL N, N-dimethylformamide (DMF), and add 11.8g (0.295mmol) NaH ( 60%), stirred for 30 minutes. Add 48.7g (0.394mol) dimethylaminothioformyl chloride to the reactant, heat to 85°C and react until the reaction is complete (about 5 hours) monitored by TLC. Stop the reaction, cool the reaction liquid to room temperature, slowly pour it into water, stir for 30 minutes, filter, wash the filter cake with water three times, and dry to obtain 43.3 g of off-white solid (5), with a yield of 91.7%.
[0052] 1 H NMR (CDCl 3 ): δ3.36(s, 3H), 3.46(s, 3H), 3.91(s, 3H), 7.13(d, J=8.64Hz, 2H), 8.08(d, J=8.64Hz, 2H).
[0053] 2. Preparation of methyl 4-dimethylcarbamoylthi...
Embodiment 2~5
[0074] The mode of operation and process are carried out by the method of embodiment 1. The results are shown in Table 3.
[0075] Table 3 Examples 2-5
[0076]
Embodiment 6
[0077] The preparation of embodiment 6 4-cyanothiobenzene trifluoride (I-6)
[0078]
[0079] 1. Preparation of 4-(dimethylaminothioformyloxy)benzonitrile (10)
[0080] In a 1000mL single-necked flask equipped with magnetic stirring, spherical condenser and drying tube, add 30g (0.252mol) p-cyanophenol, add 300mL N, N-dimethylformamide (DMF) to dissolve, and cool in an ice-salt bath 15.1 g (0.378 mol) NaH (60%) was added in portions and stirred for 30 minutes. Add 62.2 g (0.503 mol) of dimethylthiocarbamoyl chloride to the reaction solution, heat to 85° C., and monitor by TLC until the reaction is complete. Heating was stopped, the reaction liquid was cooled to room temperature, slowly added to 1.2 L of water, stirred for 30 minutes, filtered, the filter cake was washed three times with water, and recrystallized from ethanol to obtain 41.5 g of off-white solid (10), yield 86.1%.
[0081] 1 H NMR (CDCl 3 ): δ 3.36 (s, 3H), 3.45 (s, 3H), 7.18 (d, J=8.52Hz, 2H), 7.70 (d, J...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com